
a)
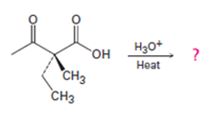
Interpretation:
Whether the
Concept introduction:
The decarboxylation of a β- keto acid takes place through the formation of a planar enol intermediate which then tautomerizes to the keto form. If the β- keto acid is optically active and if the chiral centre is involved in the reaction, the ketone product will be racemic and optically inactive. If the chiral centre is not involved in the reaction then the optical activity is retained in the ketone product.
To state:
Whether the ketone obtained by the decarboxylation of the optically active β- keto acid will be optically active or not.
To propose:
A mechanism to explain the formation of the ketone.
b)
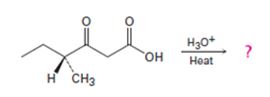
Interpretation:
Whether the ketone obtained by the decarboxylation of the optically active β- keto acid will be optically active or not is to be stated. A mechanism to explain the formation of the ketone is to be proposed.
Concept introduction:
The decarboxylation of a β- keto acid takes place through the formation of a planar enol intermediate which then tautomerizes to the keto form. If the β- keto acid is optically active and if the chiral centre is involved in the reaction, the ketone product will be racemic and optically inactive. If the chiral centre is not involved in the reaction then the optical activity is retained in the ketone product.
To state:
Whether the ketone obtained by the decarboxylation of the optically active β- keto acid will be optically active or not.
To propose:
A mechanism to explain the formation of the ketone.
Trending nowThis is a popular solution!

Chapter 22 Solutions
Organic Chemistry - With Access (Custom)
- A mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forwardIs the molecule chiral, meso, or achiral? CI .CH3 H₂C CIarrow_forwardPLEASE HELP ! URGENT!arrow_forward
- Identify priority of the substituents: CH3arrow_forwardHow many chiral carbons are in the molecule? OH F CI Brarrow_forwardA mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning



