
Organic Chemistry
7th Edition
ISBN: 9780321803221
Author: Paula Y. Bruice
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 22.1, Problem 1P
- a. Explain why, when the imidazole ring of histidine is protonated, the double-bonded nitrogen is the nitrogen that accepts the proton.
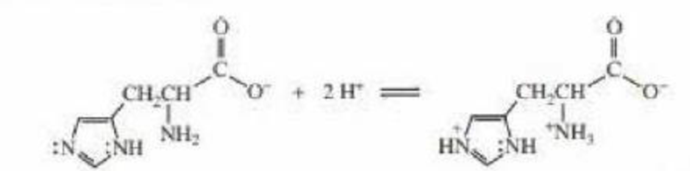
- b. Explain why. when the guanidino group of arginine is protonated, the double-bonded nitrogen is the nitrogen that accepts the proton.

Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Write the systematic name of each organic molecule:
structure
name
×
HO
OH
☐
OH
CI
CI
O
CI
OH
OH
く
Check the box under each a amino acid.
If there are no a amino acids at all, check the "none of them" box under the table.
Note for advanced students: don't assume every amino acid shown must be found in nature.
COO
H3N-C-H
CH2
HO
CH3
NH3 O
CH3-CH
CH2
OH
Onone of them
Explanation
Check
+
H3N
O
0.
O
OH
+
NH3
CH2
CH3-CH
H2N C-COOH
H
O
HIC
+
C=O
H3N-C-O
CH3- - CH
CH2
OH
Х
2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Acces
Write the systematic name of each organic molecule:
structure
HO-C-CH2-CH3
O
-OH
CH3-CH2-CH2-CH2-CH2-C-OH
CH3
CH3-CH-CH2-C-OH
Explanation
Check
S
name
Chapter 22 Solutions
Organic Chemistry
Ch. 22.1 - a. Explain why, when the imidazole ring of...Ch. 22.2 - a. Which isomer(R)-alanine or (S)-alanineis...Ch. 22.2 - Prob. 4PCh. 22.3 - Prob. 5PCh. 22.3 - Prob. 6PCh. 22.3 - Draw the predominant form for glutamate in a...Ch. 22.3 - a. Why is the pKa of the glutamate side chain...Ch. 22.4 - Explain why the pI of lysine is the average of the...Ch. 22.4 - Calculate the pI of each of the following amino...Ch. 22.4 - a. Which amino acid has the lowest pI value? b....
Ch. 22.4 - Prob. 13PCh. 22.5 - What aldehyde is formed when valine is treated...Ch. 22.5 - Prob. 15PCh. 22.5 - Prob. 16PCh. 22.5 - Prob. 17PCh. 22.5 - Prob. 18PCh. 22.6 - Why is excess ammonia used in the preceding...Ch. 22.6 - Prob. 20PCh. 22.6 - What amino acid is formed using the...Ch. 22.6 - Prob. 22PCh. 22.6 - What amino acid is formed when the aldehyde used...Ch. 22.7 - Esterase is an enzyme that catalyzes the...Ch. 22.8 - Draw the tetrapeptide Ala-Thr-Asp-Asn and indicate...Ch. 22.8 - Prob. 26PCh. 22.8 - Prob. 27PCh. 22.8 - Which bonds in the backbone of a peptide can...Ch. 22.9 - What is the configuration about each of the...Ch. 22.9 - Glutathione is a tripeptide whose function is to...Ch. 22.10 - What dipeptides would be formed by heating a...Ch. 22.10 - Suppose you are trying to synthesize the dipeptide...Ch. 22.10 - Show the steps in the synthesis of the...Ch. 22.10 - a. Calculate the overall yield of bradykinin when...Ch. 22.11 - Show the steps in the synthesis of the...Ch. 22.13 - Prob. 36PCh. 22.13 - In determining the primary structure of insulin,...Ch. 22.13 - A decapeptide undergoes partial hydrolysis to give...Ch. 22.13 - Explain why cyanogen bromide does not cleave on...Ch. 22.13 - Indicate the peptides produced from cleavage by...Ch. 22.13 - Prob. 42PCh. 22.13 - Three peptides were obtained from a trypsin...Ch. 22.14 - Prob. 44PCh. 22.15 - How would a protein that resides in the nonpolar...Ch. 22.16 - a. Which would have the greatest percentage of...Ch. 22 - Prob. 47PCh. 22 - Glycine has pK2 values of 2.34 and 9.60. At what...Ch. 22 - Prob. 49PCh. 22 - Prob. 50PCh. 22 - Aspartame (its structure is on page 1007) has a pl...Ch. 22 - Draw the form of aspartate that predominates at...Ch. 22 - A professor was preparing a manuscript for...Ch. 22 - Prob. 54PCh. 22 - Determine the amino acid sequence of a polypeptide...Ch. 22 - Prob. 56PCh. 22 - Which is the more effective buffer at...Ch. 22 - Identify the location and type of charge on the...Ch. 22 - Draw the product obtained when a lysine side chain...Ch. 22 - After the polypeptide shown below was treated with...Ch. 22 - Treatment of a polypeptide with 2-mercaptoethanol...Ch. 22 - Show how aspartame can be synthesized using DCCD.Ch. 22 - -Amino acids can be prepared by treating an...Ch. 22 - Reaction of a polypeptide with carboxypeptidase A...Ch. 22 - a. How many different octapeptides can be made...Ch. 22 - Glycine has pKa values of 2.3 and 9.6. Do you...Ch. 22 - A mixture of 15 amino acids gave the fingerprint...Ch. 22 - Write the mechanism for the reaction of an amino...Ch. 22 - Prob. 69PCh. 22 - Show how valine can be prepared by a. a...Ch. 22 - A chemist wanted to test his hypothesis that the...Ch. 22 - Propose a mechanism for the rearrangement of the...Ch. 22 - A normal polypeptide and a mutant of the...Ch. 22 - Determine the amino acid sequence of a polypeptide...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- theres 2 productsarrow_forwardDraw the major product of this solvolysis reaction. Ignore any inorganic byproducts. + CH3CH2OH Drawing Q Atoms, Bonds and Rings OCH2CH3 || OEt Charges OH 00-> | Undo Reset | Br Remove Done Drag To Pan +arrow_forwardDraw the major product of this SN1 reaction. Ignore any inorganic byproducts. CH3CO2Na CH3CO2H Drawing + Br Q Atoms, Bonds and Rings OAC Charges OH ОАс Na ဂ Br Undo Reset Remove Done Drag To Pan +arrow_forward
- Organic Functional Groups entifying positions labeled with Greek letters in acids and derivatives 1/5 ssible, replace an H atom on the a carbon of the molecule in the drawing area with a ce an H atom on the ẞ carbon with a hydroxyl group substituent. ne of the substituents can't be added for any reason, just don't add it. If neither substi er the drawing area. O H OH Oneither substituent can be added. Check D 1 Accessibility ado na witharrow_forwardDifferentiate between electrophilic and nucleophilic groups. Give examples.arrow_forwardAn aldehyde/ketone plus an alcohol gives a hemiacetal, and an excess of alcohol gives an acetal. The reaction is an equilibrium; in aldehydes, it's shifted to the right and in ketones, to the left. Explain.arrow_forward
- Draw a Haworth projection or a common cyclic form of this monosaccharide: H- -OH H- OH H- -OH CH₂OHarrow_forwardAnswer the question in the first photoarrow_forwardGgggffg2258555426855 please don't use AI Calculate the positions at which the probability of a particle in a one-dimensional box is maximum if the particle is in the fifth energy level and in the eighth energy level.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Biomolecules - Protein - Amino acids; Author: Tutorials Point (India) Ltd.;https://www.youtube.com/watch?v=ySNVPDHJ0ek;License: Standard YouTube License, CC-BY