
Concept explainers
Interpretation: Lactose is a disaccharide sugar present in milk. Lactose is composed of two monosaccharides, galactose, and glucose, that are joined together by a b-glycosidic bond. The b-glycosidic bond forms between the hydroxyl group on C- 1 of galactose and C-4 of glucose. When lactose is digested, the glycosidic bond between galactose and glucose is hydrolysed. The enzyme responsible for hydrolyzing lactose is lactase, which is found in the small intestine. Millions of people lack sufficient levels of lactase and as a result they experience lactose intolerance. If lactose is not hydrolyzed, it remains in the intestines.
Draw the structure of lactose. (The structures of galactose and glucose are shown on page 1037 with their carbon numbering scheme.)
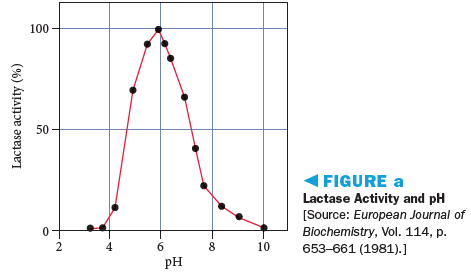
Lactase enzyme activity, like most enzymes, is sensitive to pH. Figure a illustrates how lactase activity is affected by the pH of a solution. Based on the data in Figure a, what can you conclude about the pH of the small intestine?
A glutamic acid in the active site of lactase is suspected to be involved in the catalytic mechanism. Draw the structure of the glutamic acid side chain in the ionization state that likely exists when lactase is most catalytically active.
Concept Introduction:
We know that the pH has a range from 0 to 14 and this scale is used to figure out whether the solution is acidic or basic. Solutions that have a pH of 7 are considered neutral whereas those having pH below 7 are acidic and the ones having a pH more than 7 are basic. As we see from the graph, the activity is maximum for a pH of 6. Thus, lactose should be able to work best at pH values close to 6 and any deviations for the same will result in the activity of the enzymes getting affected.
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
Chemistry: A Molecular Approach, Books a la Carte Edition (4th Edition)
- help 20arrow_forwardProvide the drawing of the unknown structure that corresponds with this data.arrow_forward20.44 The Diels-Alder reaction is not limited to making six-membered rings with only car- bon atoms. Predict the products of the following reactions that produce rings with atoms other than carbon in them. OCCH OCCH H (b) CH C(CH₂)s COOCH མ་ནས་བ (c) N=C H -0.X- (e) H C=N COOCHS + CH2=CHCH₂ →→arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





