
How many oxygen atoms are bonded to the carbon of the carbonyl of the ester functional group?
(a) None
(b) One
(c) Two
(d) Three
The correct option for the statement “How many oxygen atoms are bonded to the carbon of the carbonyl of the ester functional group?”
Answer to Problem 8RAT
The correct option for the statement “How many oxygen atoms are bonded to the carbon of the carbonyl of the ester functional group?” is option (c).
Explanation of Solution
The ester functional group contains the formula
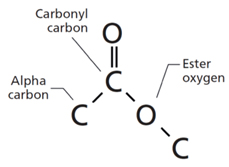
Fig.(1)
In the above structure, the number of oxygen atom present is two.
The carbonyl carbon atom is mainly bonded one oxygen atom through double bon and another oxygen atom through single bond.
Thus, two oxygen atoms are bonded to carbon of the carbonyl of the ester functional group.
Conclusion:
Therefore, the correct option for the statement “How many oxygen atoms are bonded to the carbon of the carbonyl of the ester functional group?” is option (c).
Chapter 22 Solutions
Conceptual Physical Science Explorations
Additional Science Textbook Solutions
Cosmic Perspective Fundamentals
Microbiology: An Introduction
Campbell Biology: Concepts & Connections (9th Edition)
Microbiology: An Introduction
Campbell Biology (11th Edition)
Human Biology: Concepts and Current Issues (8th Edition)
- 3arrow_forwardSet ба ||Axl 49.32 6b 71 Ay 22 Magnitude of A Angle of A 24.04 Angle of -A 22 54 155.96 ° (pos Ax) 204.04 ° (neg Ax) 335.96 ° (pos Ax) ° (neg Ax) 115.77 ° (pos Ax) 295.77 ° (pos Ax) -39 81 208.78 ° (neg Ax) 28.78 ° (neg Ax)arrow_forward3AA . not sure what i am getting wrongarrow_forward
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON





