
(a)
Interpretation: The monomers of the given
Concept introduction: The polymers (repeating structural units) are derived from the simple and reactive molecules, called as monomers. Depending upon the mode of
(a)
Answer to Problem 72E
Answer
The monomer is vinyl fluoride. It is an
Explanation of Solution
Explanation
To determine: The monomer of the given polymer.
The monomer is vinyl fluoride. It is an addition polymer whose structure is shown in Figure 1.
The structure of the monomer is,
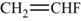
Figure 1
The polymer contains a repeating chain of vinyl fluoride, which on adding polymerization forms a polymer known as polyvinyl fluoride. Hence, the monomer is vinyl fluoride.
(b)
Interpretation: The monomers of the given polymers, their classification (condensation or addition polymers) and copolymers are to be stated.
Concept introduction: The polymers (repeating structural units) are derived from the simple and reactive molecules, called as monomers. Depending upon the mode of polymerization, polymerization mainly occurs by the addition and condensation polymerization reactions. Copolymers are defined as polymers in which repeating structural units are derived from two or more types of monomers.
(b)
Answer to Problem 72E
Answer
The monomer is
Explanation of Solution
Explanation
To determine: The monomer of the given polymer.
The monomer is
The structure of the monomer is,
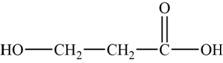
Figure 2
The polymer contains a repeating chain of
(c)
Interpretation: The monomers of the given polymers, their classification (condensation or addition polymers) and copolymers are to be stated.
Concept introduction: The polymers (repeating structural units) are derived from the simple and reactive molecules, called as monomers. Depending upon the mode of polymerization, polymerization mainly occurs by the addition and condensation polymerization reactions. Copolymers are defined as polymers in which repeating structural units are derived from two or more types of monomers.
(c)
Answer to Problem 72E
Answer
The monomers are
Explanation of Solution
Explanation
To determine: The monomer of the given polymer.
The monomers are
The structures of the monomers are,
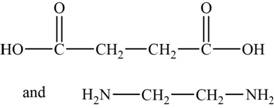
Figure 3
The polymer chain consists of the repeating units of ethane on which carboxylic group is present on carbon 1 and 2 respectively indicates that one monomer is
(d)
Interpretation: The monomers of the given polymers, their classification (condensation or addition polymers) and copolymers are to be stated.
Concept introduction: The polymers (repeating structural units) are derived from the simple and reactive molecules, called as monomers. Depending upon the mode of polymerization, polymerization mainly occurs by the addition and condensation polymerization reactions. Copolymers are defined as polymers in which repeating structural units are derived from two or more types of monomers.
(d)
Answer to Problem 72E
Answer
The monomer is
Explanation of Solution
Explanation
To determine: The monomer of the given polymer.
The monomer is
The structure of the monomer is,
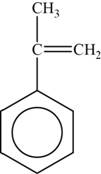
Figure 4
The repeating unit of polymer has
(e)
Interpretation: The monomers of the given polymers, their classification (condensation or addition polymers) and copolymers are to be stated.
Concept introduction: The polymers (repeating structural units) are derived from the simple and reactive molecules, called as monomers. Depending upon the mode of polymerization, polymerization mainly occurs by the addition and condensation polymerization reactions. Copolymers are defined as polymers in which repeating structural units are derived from two or more types of monomers.
(e)
Answer to Problem 72E
Answer
The monomer is
Explanation of Solution
Explanation
To determine: The monomer of the given polymer.
The monomer is
The structure of the monomer is,
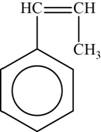
Figure 5
The repeating unit of polymer has
(f)
Interpretation: The monomers of the given polymers, their classification (condensation or addition polymers) and copolymers are to be stated.
Concept introduction: The polymers (repeating structural units) are derived from the simple and reactive molecules, called as monomers. Depending upon the mode of polymerization, polymerization mainly occurs by the addition and condensation polymerization reactions. Copolymers are defined as polymers in which repeating structural units are derived from two or more types of monomers.
(f)
Answer to Problem 72E
Answer
The monomer are
Explanation of Solution
Explanation
To determine: The monomer of the given polymer.
The monomer are and
The structures of the monomers are,
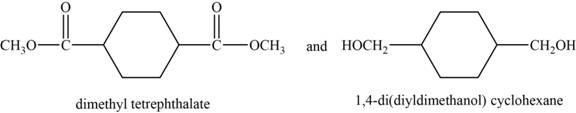
Figure 6
DMT is an organic compound. It is the diester formed from
Want to see more full solutions like this?
Chapter 22 Solutions
Lab Manual For Zumdahl/zumdahl's Chemistry, 9th
- Draw the Michael Adduct and the final product of the Robinson annulation reaction. Ignore inorganic byproducts.arrow_forwardDraw the Michael adduct and final product of the Robinson annulation reaction. Ignore inorganic byproductsarrow_forwardPost Lab Questions. 1) Draw the mechanism of your Diels-Alder cycloaddition. 2) Only one isomer of product is formed in the Diels-Alder cycloaddition. Why? 3) Imagine that you used isoprene as diene - in that case you don't have to worry about assigning endo vs exo. Draw the "endo" and "exo" products of the Diels-Alder reaction between isoprene and maleic anhydride, and explain why the distinction is irrelevant here. 4) This does not hold for other dienes. Draw the exo and endo products of the reaction of cyclohexadiene with maleic anhydride. Make sure you label your answers properly as endo or exo. 100 °C Xylenes ??? 5) Calculate the process mass intensity for your specific reaction (make sure to use your actual amounts of reagent).arrow_forward
- Indicate the product(s) A, B C and D that are formed in the reaction: H + NH-NH-CH [A+B] [C+D] hydrazonesarrow_forwardHow can you prepare a 6 mL solution of 6% H2O2, if we have a bottle of 30% H2O2?arrow_forwardHow many mL of H2O2 from the 30% bottle must be collected to prepare 6 mL of 6% H2O2.arrow_forward
- Indicate the product(s) B and C that are formed in the reaction: HN' OCH HC1 B + mayoritario C minoritario OCH3arrow_forwardIndicate the product(s) that are formed in the reaction: NH-NH, OCH3 -H₂O OCH3arrow_forward21.38 Arrange the molecules in each set in order of increasing acidity (from least acidic to most acidic). OH OH SH NH2 8 NH3 OH (b) OH OH OH (c) & & & CH3 NO2 21.39 Explain the trends in the acidity of phenol and the monofluoro derivatives of phenol. OH OH OH OH PK 10.0 PK 8.81 PK 9.28 PK 9.81arrow_forward
- identify which spectrum is for acetaminophen and which is for phenacetinarrow_forwardThe Concept of Aromaticity 21.15 State the number of 2p orbital electrons in each molecule or ion. (a) (b) (e) (f) (c) (d) (h) (i) DA (k) 21.16 Which of the molecules and ions given in Problem 21.15 are aromatic according to the Hückel criteria? Which, if planar, would be antiaromatic? 21.17 Which of the following structures are considered aromatic according to the Hückel criteria? ---0-0 (a) (b) (c) (d) (e) (h) H -H .8.0- 21.18 Which of the molecules and ions from Problem 21.17 have electrons donated by a heteroatom?arrow_forward1. Show the steps necessary to make 2-methyl-4-nonene using a Wittig reaction. Start with triphenylphosphine and an alkyl halide. After that you may use any other organic or inorganic reagents. 2. Write in the product of this reaction: CH3 CH₂ (C6H5)₂CuLi H₂O+arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





