
ORGANIC CHEMISTRY-STUDY GDE./SOL.MAN.
6th Edition
ISBN: 9780072397475
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 22, Problem 62P
Devise a synthesis of each compound from
a. 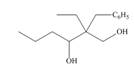 b.
b. 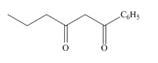
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
2) (4 pt) After the reaction was completed, the student collected the following data. Crude
product data is the data collected after the reaction is finished, but before the product
is purified. "Pure" product data is the data collected after attempted purification using
recrystallization.
Student B's data:
Crude product data
"Pure"
product data
after
recrystallization
Crude mass: 0.93 g grey solid
Crude mp: 96-106 °C
Crude % yield:
Pure mass: 0.39 g white solid
Pure mp: 111-113 °C
Pure % yield:
a) Calculate the crude and pure percent yields for the student's reaction.
b) Summarize what is indicated by the crude and pure melting points.
Don't used hand raiting
Don't used hand raiting
Chapter 22 Solutions
ORGANIC CHEMISTRY-STUDY GDE./SOL.MAN.
Ch. 22.1 - Prob. 1PCh. 22.1 - Prob. 2PCh. 22.1 - Problem 24.3
What unsaturated carbonyl compound is...Ch. 22.1 - Prob. 4PCh. 22.1 - Prob. 5PCh. 22.2 - Prob. 6PCh. 22.2 - Problem 24.7
Draw the products formed in each...Ch. 22.4 - Prob. 15PCh. 22.4 - Prob. 16PCh. 22.5 - Problem 24.16
What ester is formed when each...
Ch. 22.6 - Prob. 18PCh. 22.6 - Prob. 19PCh. 22.6 -
Draw the products of each reaction.
a. b.
Ch. 22.6 - Problem 24.20
Two steps in a synthesis of the...Ch. 22.7 - Prob. 22PCh. 22.8 - Problem 24.22
Which of the following compounds can...Ch. 22.8 - Prob. 24PCh. 22.8 - Problem 24.24
What starting materials are needed...Ch. 22 - Prob. 29PCh. 22 - 24.29 What steps are needed to convert A to B?
Ch. 22 - Prob. 31PCh. 22 - 24.31 Draw the product formed in each directed...Ch. 22 - Prob. 33PCh. 22 - 24.33 What starting materials are needed to...Ch. 22 - Prob. 35PCh. 22 - Prob. 36PCh. 22 - 24.36 Identify the structures of C and D in the...Ch. 22 - Prob. 38PCh. 22 - Prob. 39PCh. 22 - 24.39 Draw the product formed from a Claisen...Ch. 22 - Prob. 41PCh. 22 - 24.41 Even though B contains three ester groups, a...Ch. 22 - Prob. 43PCh. 22 - Prob. 44PCh. 22 - 24.44 Vetivone is isolated from vetiver, a...Ch. 22 - Draw the product of each Robinson annulation from...Ch. 22 - Prob. 50PCh. 22 - Prob. 51PCh. 22 - 24.52 Draw a stepwise mechanism for the following...Ch. 22 - Prob. 53PCh. 22 - Prob. 54PCh. 22 - Prob. 55PCh. 22 - Prob. 56PCh. 22 - Prob. 57PCh. 22 - Prob. 58PCh. 22 - Prob. 59PCh. 22 - 24.62 Devise a synthesis of each compound from ,...Ch. 22 - Prob. 63P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Don't used hand raitingarrow_forwardShown below is the major resonance structure for a molecule. Draw the second best resonance structure of the molecule. Include all non-zero formal charges. H. H. +N=C H H H Cl: Click and drag to start drawing a structure. : ? g B S olo Ar B Karrow_forwardDon't used hand raitingarrow_forward
- S Shown below is the major resonance structure for a molecule. Draw the second best resonance structure of the molecule. Include all non-zero formal charges. H H = HIN: H C. :0 H /\ H H Click and drag to start drawing a structure. ×arrow_forwardPlease help me figure out these calculation and what should be plotted. These are notes for my chemistry class.arrow_forwardNonearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY