
EBK ORGANIC CHEMISTRY
12th Edition
ISBN: 9781119233664
Author: Snyder
Publisher: VST
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 22, Problem 44P
The following reaction sequence represents an elegant method of synthesis of 2-deoxy-D-ribose, IV, published by D. C. C. Smith in 1955:
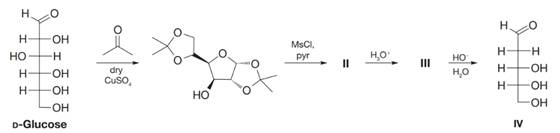
(a) What are the structures of II and III?
(b) Propose a mechanism for the conversion of III to IV.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
An electrode process takes place at a metal-solution interface. Indicate the current condition that must be met for Faradaic rectification to occur.
At a metal-solution interface, an electron is exchanged, and the symmetry factor beta < 0.5 is found in the Butler-Volmer equation. What does this indicate?
Topic: Photochemistry and Photophysics of Supramolecules
Chapter 22 Solutions
EBK ORGANIC CHEMISTRY
Ch. 22 - Prob. 1PPCh. 22 - Prob. 2PPCh. 22 - Prob. 3PPCh. 22 - Prob. 4PPCh. 22 - Prob. 5PPCh. 22 - Prob. 6PPCh. 22 - Prob. 7PPCh. 22 - Prob. 8PPCh. 22 - Practice Problem 22.9 What products would you...Ch. 22 - Prob. 10PP
Ch. 22 - Prob. 11PPCh. 22 - Prob. 12PPCh. 22 - Prob. 13PPCh. 22 - Prob. 14PPCh. 22 - Prob. 15PPCh. 22 - Prob. 16PPCh. 22 - Prob. 17PPCh. 22 - Prob. 18PPCh. 22 - Prob. 19PPCh. 22 - Prob. 20PCh. 22 - Prob. 21PCh. 22 - Prob. 22PCh. 22 - Prob. 23PCh. 22 - Prob. 24PCh. 22 - Prob. 25PCh. 22 - Prob. 26PCh. 22 - Prob. 27PCh. 22 - Prob. 28PCh. 22 - Prob. 29PCh. 22 - Prob. 30PCh. 22 - Prob. 31PCh. 22 - Prob. 32PCh. 22 - Prob. 33PCh. 22 - Prob. 34PCh. 22 - Prob. 35PCh. 22 - Prob. 36PCh. 22 - Prob. 37PCh. 22 - Prob. 38PCh. 22 - Arbutin, a compound that can be isolated from the...Ch. 22 - Prob. 40PCh. 22 - Prob. 41PCh. 22 - Prob. 42PCh. 22 - Prob. 43PCh. 22 - 22.44 The following reaction sequence represents...Ch. 22 - 22.45
The NMR data for the two anomers...Ch. 22 - Shikimic acid is a key biosynthetic intermediate...
Additional Science Textbook Solutions
Find more solutions based on key concepts
66. A flutist assembles her flute in a room where the speed of sound is 342 m/s. When she plays the note A, it ...
College Physics: A Strategic Approach (3rd Edition)
For the arrangement of forces in Problem 81, a 2.00 kg particle is released at x = 5.00 m with an initial veloc...
Fundamentals of Physics Extended
The genotype of F1, individuals in a tetrahybrid cross is AaBbCcDd. Assuming lndependent assortment of these fo...
Campbell Biology (11th Edition)
Where is transitional epithelium found and what is its importance at those sites?
Anatomy & Physiology (6th Edition)
Use the following graph to answer questions 3 and 4. 3. Which of the lines best depicts the log phase of a ther...
Microbiology: An Introduction
31. Which changes in pregnancy have an effect on the ability to exercise?
Principles of Anatomy and Physiology
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- When two solutions, one of 0.1 M KCl (I) and the other of 0.1 M MCl (II), are brought into contact by a membrane. The cation M cannot cross the membrane. At equilibrium, x moles of K+ will have passed from solution (I) to (II). To maintain the neutrality of the two solutions, x moles of Cl- will also have to pass from I to II. Explain this equality: (0.1 - x)/x = (0.1 + x)/(0.1 - x)arrow_forwardCalculate the variation in the potential of the Pt/MnO4-, Mn2+ pair with pH, indicating the value of the standard potential. Data: E0 = 1.12.arrow_forwardGiven the cell: Pt l H2(g) l dis X:KCl (sat) l Hg2Cl2(s) l Hg l Pt. Calculate the emf of the cell as a function of pH.arrow_forward
- The decimolar calomel electrode has a potential of 0.3335 V at 25°C compared to the standard hydrogen electrode. If the standard reduction potential of Hg22+ is 0.7973 V and the solubility product of Hg2Cl2 is 1.2x 10-18, find the activity of the chlorine ion at this electrode.Data: R = 8.314 J K-1 mol-1, F = 96485 C mol-1, T = 298.15 K.arrow_forward2. Add the following group of numbers using the correct number of significant figures for the answer. Show work to earn full credit such as rounding off the answer to the correct number of significant figures. Replace the question marks with the calculated answers or write the calculated answers near the question marks. 10916.345 37.40832 5.4043 3.94 + 0.0426 ? (7 significant figures)arrow_forwardThe emf at 25°C of the cell: Pt l H2(g) l dis X:KCl (sat) l Hg2Cl2(s) l Hg l Pt was 612 mV. When solution X was replaced by normal phosphate buffer solution with a pH of 6.86, the emf was 741 mV. Calculate the pH of solution X.arrow_forward
- Indicate how to calculate the potential E of the reaction Hg2Cl2(s) + 2e ⇄ 2Hg + 2Cl- as a function of the concentration of Cl- ions. Data: the solubility product of Hg2Cl2.arrow_forwardHow can Beer’s Law be used to determine the concentration in a selected food sample. Provide an in-depth discussion and examples of this.arrow_forwardb) H3C- H3C Me CH 3 I HN Me H+arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License