
Organic Chemistry (8th Edition)
8th Edition
ISBN: 9780134042282
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 22, Problem 41P
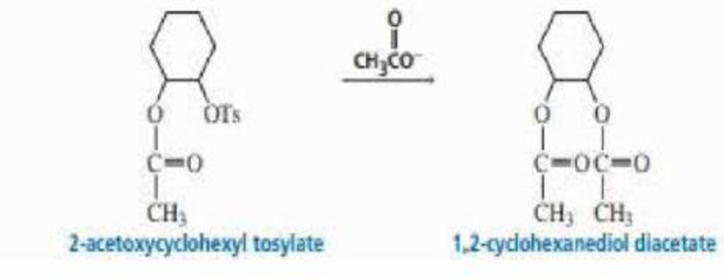
2-Acetoxycyclohexyl tosylate reacts with acetate ion to form 1,2-cyclohexanediol diacetate. The reaction is stereospecific—that is, the stereoisomers obtained as products depend on the stereoisomer used as a reactant. Recall that because 2-acetoxycyclohexyl tosylate has two asymmetric centers, it has four stereoisomers—two are cis and two are trans. Explain the following observations:
- a. Both cis reactants form an optically active trans product, but each cis reactant forms a different trans product.
- b. Both trans reactants form the same racemic mixture.
- c. A trans reactant is more reactive than a cis reactant.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
State two variables on which the transport number in electrochemistry depends.
Among the methods for measuring transport numbers are the Hittorf method and the moving surface method. Let's explain them briefly.
can you help me solve and highlight these hw
Chapter 22 Solutions
Organic Chemistry (8th Edition)
Ch. 22.2 - Compare each of the mechanisms listed here with...Ch. 22.2 - Prob. 3PCh. 22.2 - Prob. 4PCh. 22.3 - a. Draw the mechanism for the following reaction...Ch. 22.5 - Prob. 7PCh. 22.5 - Propose a mechanism for the Co2+ catalyzed...Ch. 22.6 - Prob. 9PCh. 22.7 - Prob. 10PCh. 22.7 - Prob. 12PCh. 22.7 - Prob. 13P
Ch. 22.9 - Which of the following amino acid side chains can...Ch. 22.9 - Which of the following C-terminal peptide bonds is...Ch. 22.9 - Carboxypeptidase A has esterase activity as well...Ch. 22.10 - Arginine and lysine side chains fit into trypsins...Ch. 22.10 - Explain why serine proteases do not catalyze...Ch. 22.11 - If H2 18O is used in the hydrolysis reaction...Ch. 22.11 - Draw the pH-activity profile for an enzyme that...Ch. 22.12 - The pHactivity profile for glucose-6-phosphate...Ch. 22.12 - Prob. 23PCh. 22.13 - Draw the mechanism for the hydroxide ion-catalyzed...Ch. 22.13 - What advantage does the enzyme gain by forming an...Ch. 22.13 - Prob. 26PCh. 22.13 - Prob. 27PCh. 22.13 - Aldolase shows no activity if it is incubated with...Ch. 22 - Which of the following parameters would be...Ch. 22 - Prob. 29PCh. 22 - Prob. 30PCh. 22 - Prob. 31PCh. 22 - Indicate the type of catalysis that is occurring...Ch. 22 - The deuterium kinetic isotope effect (KH2O/KD2O)...Ch. 22 - Prob. 34PCh. 22 - Co2+ catalyzes the hydrolysis of the lactam shown...Ch. 22 - there are two kinds of aldolases. Class I...Ch. 22 - Prob. 37PCh. 22 - The hydrolysis of the ester shown here is...Ch. 22 - Prob. 39PCh. 22 - At pH = 12, the rate of hydrolysis of ester A is...Ch. 22 - 2-Acetoxycyclohexyl tosylate reacts with acetate...Ch. 22 - Proof that an imine was formed between aldolase...Ch. 22 - Prob. 43PCh. 22 - a. Explain why the alkyl halide shown here reacts...Ch. 22 - Triosephosphate isomerase (TIM) catalyzes the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 10. Stereochemistry. Assign R/S stereochemistry for the chiral center indicated on the following compound. In order to recieve full credit, you MUST SHOW YOUR WORK! H₂N CI OH CI カー 11. () Stereochemistry. Draw all possible stereoisomers of the following compound. Assign R/S configurations for all stereoisomers and indicate the relationship between each as enantiomer, diastereomer, or meso. NH2 H HNH, -18arrow_forwardb) 8. Indicate whether the following carbocation rearrangements are likely to occur Please explain your rational using 10 words or less not likely to occur • The double bond is still in the Same position + Likely to oc occur WHY? -3 H3C Brave Chair Conformers. Draw the chair conformer of the following substituted cyclohexane. Peform a RING FLIP and indicate the most stable conformation and briefly explain why using 20 words or less. CI 2 -cobs ?? MUST INDICATE H -2 -2 Br EQ Cl OR AT Br H& most stable WHY? - 4arrow_forwardCH 12 Conformational Analysis. Draw all 6 conformers (one above each letter) of the compound below looking down the indicated bond. Write the letter of the conformer with the HIGHEST and LOWEST in energies on the lines provided. NOTE: Conformer A MUST be the specific conformer of the structure as drawn below -4 NOT HOH OH 3 Conformer A: Br OH A Samo Br H 04 Br H H3 CH₂ H anti stagere Br CH clipsed H Brott H IV H MISSING 2 -2 B C D E F X 6 Conformer with HIGHEST ENERGY: 13. (1 structure LOWEST ENERGY: Nomenclature. a) Give the systematic (IUPAC) name structure. b) Draw the corresponding to this name. HINT: Do not forget to indicate stereochemistry when applicable. a) ८८ 2 "Br {t༐B,gt)-bemn€-nehpརི་ཚ༐lnoa Parent name (noname) 4 Bromo Sub = 2-methylethyl-4 Bromo nonane b) (3R,4S)-3-chloro-4-ethyl-2,7-dimethyloctane # -2 -2arrow_forward
- in the scope of the SCH4U course! please show all steps as im still learning how to format my answers in the format given, thank you!arrow_forwardhelp me solve this HWarrow_forwardMolecules of the form AH2 can exist in two potential geometries: linear or bent. Construct molecular orbital diagrams for linear and bent CH2. Identify the relevant point group, include all of the appropriate symmetry labels and pictures, and fill in the electrons. Which geometry would you predict to be more stable, and why? (Please draw out the diagram and explain)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License