
Chemistry the Central Science 13th Edition Custom for Lamar University
13th Edition
ISBN: 9781269962667
Author: Theodore L. Brown, H. Eugene LeMay Jr., Bruce E. Bursten, Batherine J. Murphy, Patrick M. Woodward, Matthew W. Stoltzfus
Publisher: Pearson Learning Center
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 22, Problem 22E
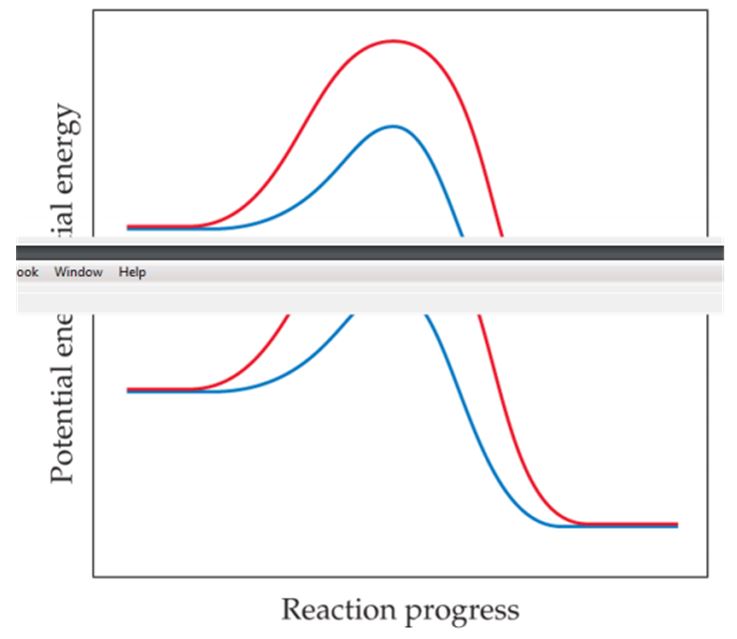
The following graph shows two different reaction pathways for the same overall reaction at the same temperature. Is each of the following statements true or false? (a) The rate is faster for the red path than for the blue path. (b) For both paths, the rate of the reverse reaction is slower than the rate of the forward reaction. (c) The energy change ΔE is the same for both paths. [Section 14.6]
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Denote the dipole for the indicated bonds in the following molecules.
H3C
✓
CH3
B
F-CCl 3
Br-Cl
H3C Si(CH3)3
wwwwwww
OH
НО.
HO
HO
OH
vitamin C
CH3
For the SN2 reaction, draw the major organic product and select the correct (R) or (S) designation around the stereocenter
carbon in the organic substrate and organic product. Include wedge-and-dash bonds and draw hydrogen on a stereocenter.
Η
1
D
EN
Select Draw Templates More
C
H
D
N
Erase
Q9: Explain why compound I is protonated on O while compound II is protonated on N.
NH2
NH2
I
II
Chapter 22 Solutions
Chemistry the Central Science 13th Edition Custom for Lamar University
Ch. 22.1 - Prob. 21.1.1PECh. 22.1 - Prob. 21.1.2PECh. 22.1 - Prob. 21.2.1PECh. 22.1 - Prob. 21.2.2PECh. 22.3 - At 25 ° C, the decomposition of dinitrogen...Ch. 22.3 - Practice Exercise 2 The decomposition of dimethyl...Ch. 22.4 - Practice Exercise 1 For a certain reaction A ...Ch. 22.4 - Prob. 21.4.2PECh. 22.7 - Prob. 21.7.1PECh. 22.7 - Prob. 21.7.2PE
Ch. 22.10 - Prob. 21.10.1PECh. 22.10 - Prob. 21.10.2PECh. 22.10 - Prob. 21.7.1PECh. 22.10 - Prob. 21.7.2PECh. 22 - Prob. 1DECh. 22 - Prob. 1ECh. 22 - Prob. 2ECh. 22 - Prob. 3ECh. 22 - Prob. 4ECh. 22 - The gas-phase reaction CL (g) + HBr (g) + HCl (g)...Ch. 22 - What is the molecularity of each of the following...Ch. 22 - Prob. 7ECh. 22 - Prob. 8ECh. 22 - Cyclopentadiene (C5H6) reacts with itself to form...Ch. 22 - Practice Exercise 1 An Alternative two-step...Ch. 22 - Prob. 11ECh. 22 - Practice Exercise 1
Consider the...Ch. 22 - Prob. 13ECh. 22 - Prob. 14ECh. 22 - Prob. 15ECh. 22 - Prob. 16ECh. 22 - You study the rate of a reaction, measuring both...Ch. 22 - Suppose that for the reaction K+L M, you monitor...Ch. 22 - Prob. 19ECh. 22 - Prob. 20ECh. 22 - Prob. 21ECh. 22 - The following graph shows two different reaction...Ch. 22 - Prob. 23ECh. 22 - Prob. 24ECh. 22 - Prob. 25ECh. 22 - Prob. 26ECh. 22 - Prob. 27ECh. 22 - Prob. 28ECh. 22 - Prob. 29ECh. 22 - Prob. 30ECh. 22 - Prob. 31ECh. 22 - Prob. 32ECh. 22 - Prob. 33ECh. 22 - Prob. 34ECh. 22 - Prob. 35ECh. 22 - Prob. 36ECh. 22 - Prob. 37ECh. 22 - Prob. 38ECh. 22 - Prob. 39ECh. 22 - Prob. 40ECh. 22 - Prob. 41ECh. 22 - Prob. 42ECh. 22 - Prob. 43ECh. 22 - Prob. 44ECh. 22 - Prob. 45ECh. 22 - Prob. 46ECh. 22 - Prob. 47ECh. 22 - Prob. 48ECh. 22 - Prob. 49ECh. 22 - Prob. 50ECh. 22 - Prob. 51ECh. 22 - Prob. 52ECh. 22 - Prob. 53ECh. 22 - Prob. 54ECh. 22 - Prob. 55ECh. 22 - Prob. 56ECh. 22 - Prob. 57ECh. 22 - Prob. 58ECh. 22 - Prob. 59ECh. 22 - Prob. 60ECh. 22 - Prob. 61ECh. 22 - Prob. 62ECh. 22 - Prob. 63ECh. 22 - Prob. 64ECh. 22 - Prob. 65ECh. 22 - Prob. 66ECh. 22 - Prob. 67ECh. 22 - Prob. 68ECh. 22 - Prob. 69ECh. 22 - Prob. 70ECh. 22 - Prob. 71ECh. 22 - Prob. 72ECh. 22 - Prob. 73ECh. 22 - Prob. 74ECh. 22 - Prob. 75ECh. 22 - Prob. 76ECh. 22 - Prob. 77ECh. 22 - Prob. 78ECh. 22 - Prob. 79ECh. 22 - Prob. 80ECh. 22 - Prob. 81AECh. 22 - Prob. 82AECh. 22 - Prob. 83AECh. 22 - Prob. 84AECh. 22 - Prob. 85AECh. 22 - Prob. 86AECh. 22 - Prob. 87AECh. 22 - Prob. 88AECh. 22 - Prob. 89AECh. 22 - Prob. 90AECh. 22 - Prob. 91AECh. 22 - Prob. 92IECh. 22 - Prob. 93IECh. 22 - Prob. 94IECh. 22 - Prob. 95IECh. 22 - Prob. 96IECh. 22 - Prob. 97IECh. 22 - Prob. 98IECh. 22 - Prob. 99IECh. 22 - Prob. 100IECh. 22 - Prob. 101IECh. 22 - Prob. 102IECh. 22 - Prob. 103IECh. 22 - Prob. 104IECh. 22 - Prob. 105IECh. 22 - Prob. 106IECh. 22 - Prob. 107IE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- AN IR spectrum, a 13 CMR spectrum, and a 1 HMR spectrum were obtained for an unknown structure with a molecular formula of C9H10. Draw the structure of this compound.arrow_forwardAN IR spectrum, a 13 CMR spectrum, and a 1 HMR spectrum were obtained for an unknown structure with a molecular formula of C9H10. Draw the structure of this compound.arrow_forward(a) What is the hybridization of the carbon in the methyl cation (CH3*) and in the methyl anion (CH3¯)? (b) What is the approximate H-C-H bond angle in the methyl cation and in the methyl anion?arrow_forward
- Q8: Draw the resonance structures for the following molecule. Show the curved arrows (how you derive each resonance structure). Circle the major resonance contributor.arrow_forwardQ4: Draw the Lewis structures for the cyanate ion (OCN) and the fulminate ion (CNO). Draw all possible resonance structures for each. Determine which form for each is the major resonance contributor.arrow_forwardIn the following molecule, indicate the hybridization and shape of the indicated atoms. CH3 N CH3 HÖ: H3C CI: ::arrow_forward
- Q3: Draw the Lewis structures for nitromethane (CH3NO2) and methyl nitrite (CH3ONO). Draw at least two resonance forms for each. Determine which form for each is the major resonance contributor.arrow_forwardQ1: Draw a valid Lewis structures for the following molecules. Include appropriate charges and lone pair electrons. If there is more than one Lewis structure available, draw the best structure. NH3 Sulfate Boron tetrahydride. C3H8 (linear isomer) OCN NO3 CH3CN SO2Cl2 CH3OH2*arrow_forwardQ2: Draw all applicable resonance forms for the acetate ion CH3COO. Clearly show all lone pairs, charges, and arrow formalism.arrow_forward
- Please correct answer and don't used hand raitingarrow_forward9. The following reaction, which proceeds via the SN1/E1 mechanisms, gives three alkene products (A, B, C) as well as an ether (D). (a) Show how each product arises mechanistically. (b) For the alkenes, determine the major product and justify your answer. (c) What clues in the reaction as shown suggest that this reaction does not go by the SN2/E2 mechanism route? (CH3)2CH-CH-CH3 CH3OH 1 Bl CH3OH ⑧· (CH3)2 CH-CH=CH2 heat H ⑥③ (CH3)2 C = C = CH3 © СнЗ-С-Снаснз сна (CH 3 ) 2 C H G H CH 3 оснзarrow_forwardPlease Don't used hand raitingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Kinetics: Chemistry's Demolition Derby - Crash Course Chemistry #32; Author: Crash Course;https://www.youtube.com/watch?v=7qOFtL3VEBc;License: Standard YouTube License, CC-BY