
A 1.00-mol sample of an ideal gas (γ = 1.40) is carried through the Carnot cycle described in Figure 22.11. At point A, the pressure is 25.0 atm and the temperature is 600 K. At point C, the pressure is 1.00 atm and the temperature is 400 K. (a) Determine the pressures and volumes at points A, B, C, and D. (b) Calculate the net work done per cycle.
(a)
The pressure and volume at points
Answer to Problem 22.81CP
For the point
Explanation of Solution
Given data: The number of mole is
Consider the figure of Carnot cycle,
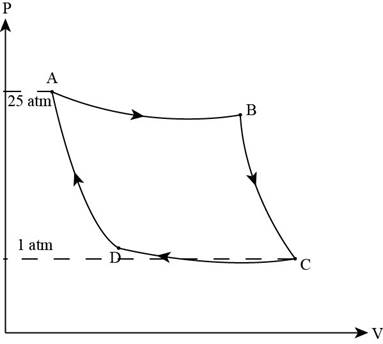
Figure (1)
From the figure, it is clear that the process from
Apply the ideal gas equation for volume at
Here,
The value of ideal gas constant is
Substitute
Thus, the volume at point
Apply the ideal gas equation for volume at
Here,
Substitute
Thus, the volume at point
For the adiabatic process:
The condition is,
Here,
Substitute
Thus, the volume of gas at point
The formula for the pressure at point
Substitute
Thus, the pressure at point
For the adiabatic process:
The condition is,
Here,
Substitute
Thus, the volume of gas at point
The formula for the pressure at point
Substitute
Conclusion:
Therefore, For the point
(b)
The net work done per cycle.
Answer to Problem 22.81CP
The net work done per cycle is
Explanation of Solution
The formula for the net work done per cycle is,
Substitute
Conclusion:
Therefore, the net work done per cycle is
Want to see more full solutions like this?
Chapter 22 Solutions
Physics for Scientists and Engineers, Technology Update, Hybrid Edition (with Enhanced WebAssign Multi-Term LOE Printed Access Card for Physics)
- Please solve this problem correctly please and be sure to provide explanation on each step so I can understand what's been done thank you. (preferrably type out everything)arrow_forwardUse a calculation to determine how far the fishing boat is from the water level .Determine distance Yarrow_forwardNo chatgpt pls will upvote Already got wrong chatgpt answerarrow_forward
- 2. 1. Tube Rating Charts Name: Directions: For the given information state if the technique is safe or unsafe and why. 60 Hertz Stator Operation Effective Focal Spot Size- 0.6 mm Peak Kilovolts MA 2 150 140 130 120 110 100 90 80 70 2501 60 50 40 30 .01 .02 .04.06 .1 .2 .4.6 1 8 10 Maximum Exposure Time In Seconds Is an exposure of 80 kVp, 0.1 second and 200 mA within the limits of the single phase, 0.6 mm focal spot tube rating chart above? Is an exposure of 100 kVp, 0.9 second and 150 mA within the limits of the single phase, 0.6 mm focal spot tube rating chart above?arrow_forwardQ: You have a CO2 laser resonator (λ = 10.6 μm). It has two curved mirrors with R₁=10m, R2= 8m, and mirror separation /= 5m. Find: R2-10 m tl Z-O 12 R1-8 m 1. Confocal parameter. b= 21w2/2 =√1 (R1-1)(R2-1)(R1+R2-21)/R1+R2-21) 2. Beam waist at t₁ & t2- 3. Waist radius (wo). 4. 5. The radius of the laser beam outside the resonator and about 0.5m from R₂- Divergence angle. 6. Radius of curvature for phase front on the mirrors R₁ & R2-arrow_forwardNo chatgpt pls will upvotearrow_forward
 Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning





