
General, Organic, and Biochemistry
10th Edition
ISBN: 9781260506198
Author: Denniston, Katherine
Publisher: MCGRAW-HILL HIGHER EDUCATION
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 22, Problem 22.49QP
Interpretation Introduction
Interpretation:
The conversion of fumarate to malate in terms of the chemistry of alcohol and
Concept Introduction:
Hydration reaction is defined as a
For example, the addition of water molecules to alkene produces alcohol. The reaction of formation of alcohol is as follows:
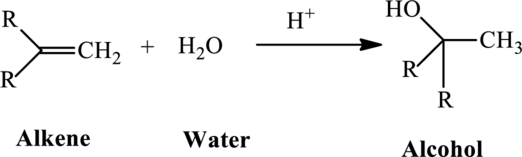
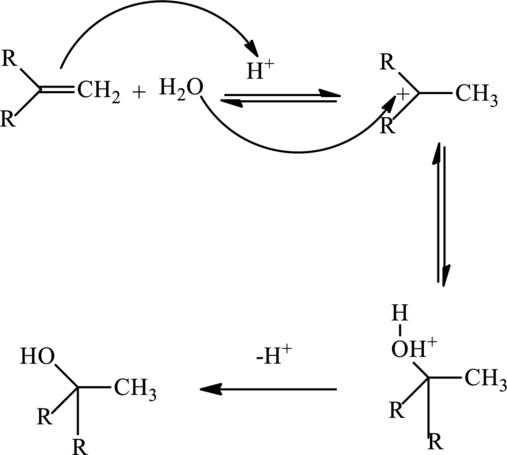
The mechanism involved in the above reaction is as follows:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What is the missing reactant in this organic reaction?
R+
OH
HD
CH3-CH2-CH-CH3
H
A
CH3
CH3-CH2-C-O-CH-CH2-CH3
+ H₂O
Specifically, in the drawing area below draw the condensed structure of R.
If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area.
No answer
Click anywhere to draw the first
atom of your structure.
☐ :
Jm
+
Draw the major product of the following E2 reaction. Make sure you pay attention to
REGIOCHEMISTRY and STEREOCHEMISTRY. Explain why this product is formed
using 10 words or less for each.
(a)
NaH
Br
acetone
TSO,
NaH
(b)
acetone
2. Circle the compound that will react SLOWER in an E2 reaction. To get credit for this
question, you must EXPLAIN how you got your answer using STRUCTURES and WORDS.
Br
**
Br...
Chapter 22 Solutions
General, Organic, and Biochemistry
Ch. 22.1 - Prob. 22.1QCh. 22.1 - How do the mitochondria differ from the other...Ch. 22.1 - Prob. 22.3QCh. 22.1 - Describe the evidence that suggests that...Ch. 22.2 - Prob. 22.5QCh. 22.2 - Prob. 22.6QCh. 22.3 - Prob. 22.7QCh. 22.3 - Prob. 22.8QCh. 22.6 - Prob. 22.9QCh. 22.6 - Write a balanced chemical equation for the...
Ch. 22.6 - Prob. 22.1PPCh. 22.7 - Prob. 22.11QCh. 22.7 - Prob. 22.12QCh. 22.8 - Prob. 22.13QCh. 22.8 - Prob. 22.14QCh. 22.9 - Prob. 22.15QCh. 22.9 - Prob. 22.16QCh. 22 - Prob. 22.17QPCh. 22 - Prob. 22.18QPCh. 22 - Prob. 22.19QPCh. 22 - Prob. 22.20QPCh. 22 - Prob. 22.21QPCh. 22 - Prob. 22.22QPCh. 22 - Prob. 22.23QPCh. 22 - Prob. 22.24QPCh. 22 - Prob. 22.25QPCh. 22 - Prob. 22.26QPCh. 22 - Prob. 22.27QPCh. 22 - Prob. 22.28QPCh. 22 - Prob. 22.29QPCh. 22 - Prob. 22.30QPCh. 22 - Prob. 22.31QPCh. 22 - Prob. 22.32QPCh. 22 - Prob. 22.33QPCh. 22 - Prob. 22.34QPCh. 22 - Prob. 22.35QPCh. 22 - Prob. 22.36QPCh. 22 - Prob. 22.38QPCh. 22 - Prob. 22.41QPCh. 22 - Prob. 22.42QPCh. 22 - Prob. 22.43QPCh. 22 - Prob. 22.44QPCh. 22 - Prob. 22.45QPCh. 22 - Prob. 22.46QPCh. 22 - Prob. 22.47QPCh. 22 - Prob. 22.48QPCh. 22 - Prob. 22.49QPCh. 22 - Prob. 22.50QPCh. 22 - Prob. 22.51QPCh. 22 - Prob. 22.52QPCh. 22 - Prob. 22.53QPCh. 22 - Prob. 22.54QPCh. 22 - To what class of enzymes does dinucleotide...Ch. 22 - Prob. 22.56QPCh. 22 - Prob. 22.57QPCh. 22 - Prob. 22.58QPCh. 22 - Explain why deficiencies of citric acid cycle...Ch. 22 - Prob. 22.60QPCh. 22 - Prob. 22.61QPCh. 22 - Prob. 22.62QPCh. 22 - Prob. 22.63QPCh. 22 - Prob. 22.64QPCh. 22 - Prob. 22.65QPCh. 22 - Prob. 22.66QPCh. 22 - Prob. 22.67QPCh. 22 - Prob. 22.68QPCh. 22 - Prob. 22.69QPCh. 22 - Prob. 22.70QPCh. 22 - Prob. 22.71QPCh. 22 - Prob. 22.72QPCh. 22 - Prob. 22.73QPCh. 22 - Prob. 22.74QPCh. 22 - Prob. 22.75QPCh. 22 - Prob. 22.76QPCh. 22 - Prob. 22.77QPCh. 22 - Prob. 22.78QPCh. 22 - Prob. 22.79QPCh. 22 - Prob. 22.80QPCh. 22 - Prob. 22.81QPCh. 22 - Prob. 22.82QPCh. 22 - Why is the glutamate family of transaminases so...Ch. 22 - Prob. 22.84QPCh. 22 - Prob. 22.85QPCh. 22 - Prob. 22.87QPCh. 22 - Prob. 22.88QPCh. 22 - Prob. 22.89QPCh. 22 - Prob. 22.90QPCh. 22 - Prob. 22.91QPCh. 22 - Prob. 22.92QPCh. 22 - Prob. 22.93QPCh. 22 - Prob. 22.94QPCh. 22 - Prob. 22.95QPCh. 22 - Prob. 22.96QPCh. 22 - Prob. 22.97QPCh. 22 - Prob. 22.98QPCh. 22 - Prob. 22.99QPCh. 22 - Prob. 22.100QPCh. 22 - Prob. 22.101QPCh. 22 - Prob. 22.102QPCh. 22 - Prob. 1MCPCh. 22 - Prob. 2MCPCh. 22 - Prob. 3MCPCh. 22 - Prob. 4MCPCh. 22 - Prob. 5MCPCh. 22 - Prob. 7MCPCh. 22 - Prob. 8MCPCh. 22 - Prob. 10MCPCh. 22 - Prob. 11MCP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 8. 2 20 00 Draw ALL of the possible products for the following reaction CIRCLE the MAJOR product NaOMe MeOHarrow_forwardNAME: 1. Draw the major product of the following E2 reaction. Make sure you pay attention to REGIOCHEMISTRY and STEREOCHEMISTRY. To get credit for this question, you must EXPLAIN how you got your answer using STRUCTURES and WORDS. Br NaOCH3 acetone F2 reaction To get credit for thisarrow_forward3. Reactions! Fill in the information missing below. Make sure to pay attention to REGIOCHEMISTRY and STEREOCHEMISTRY. Br2 CH3OH + 4. Mechanism! Show the complete arrow pushing mechanism, including all steps and intermediates for the following reactions. To get credit for this, you MUST show how ALL bonds are broken and formed, using arrows to show the movement of electrons. H3O+ HOarrow_forward
- Please provide a synthesis for the Ester using proponoic acid, thank you!arrow_forwardPlease help with the curved arrow mechanism of this reaction, thank youarrow_forwardConcentration (mg/l) Peak Area 0 158 10 10241 20 18425 30 26457 40 37125 50 44256 60 56124 Question: Determine the regression equation (a and b coefficients) from first principlesarrow_forward
- Concentration (mg/l) Peak Area 0 158 10 10241 20 18425 30 26457 40 37125 50 44256 60 56124 You have been asked to determine the concentration of citral in a highly valued magnolia essential oil. QUESTION: Calculate the concentration of citral in your highly valued magnolia essential oil which returns a peak area of 41658arrow_forwardNeed help with these problems...if you can please help me understand problems E & F.arrow_forwardPlease help me solve these problems. Thank you in advance.arrow_forward
- Predict the products of this organic reaction: O N IN A N + H2O + HCI ? Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. 田 C + Explanation Check Click and drag to start drawing a structure. C © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forward6. For each of the following, fill in the synthesis arrows with reagents and show the intermediates. You DO NOT need to use the same number of arrows that are shown (you may use more or less), but the product must be formed from the reactant. Then write the mechanism of one step in the synthesis (you can choose which step to write the mechanism for), including all reagents required, clearly labeling the nucleophile and electrophile for each step, and using curved arrows to show the steps in the mechanism. a. b. OHarrow_forwardDraw the productsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY