
Concept explainers
(a)
Interpretation:
The closest
Concept introduction:
A unit cell of the crystal is the three-dimensional arrangement of the atoms present in the crystal. The unit cell is the smallest and simplest unit of the crystal which on repetition forms an entire crystal. Unit cell can be a cubic unit cell or hexagonal unit cell. The classification of a unit cell depends on the lattice site occupied by the atoms.
Answer to Problem 22.35E
The closest
Explanation of Solution
The structure of a face-centered cubic lattice is shown below.
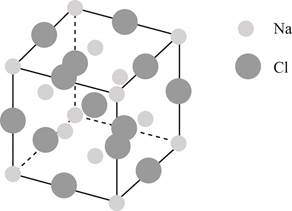
Figure 1
The plane of face-centered cubic lattice that has
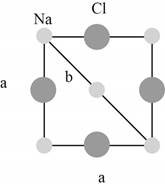
Figure 2
The closest
The lattice parameter
The Pythagoras theorem is shown below.
Where,
•
•
•
The hypotenuse of the triangle shown in Figure (2) is
The base and perpendicular of the triangle shown in Figure (2) are
Substitute the value of hypotenuse, base, and perpendicular in the equation (1).
Substitute the value of
Therefore, the closest
The closest
(b)
Interpretation:
The closest
Concept introduction:
A unit cell of the crystal is the three-dimensional arrangement of the atoms present in the crystal. The unit cell is the smallest and simplest unit of the crystal which on repetition forms an entire crystal. Unit cell can be a cubic unit cell or hexagonal unit cell. The classification of a unit cell depends on the lattice site occupied by the atoms.
Answer to Problem 22.35E
The closest
Explanation of Solution
The structure of a face-centered cubic lattice is shown below.
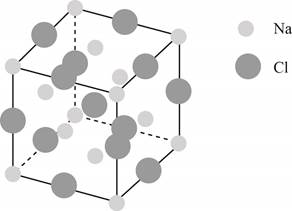
Figure 1
The plane of face-centered cubic lattice that has
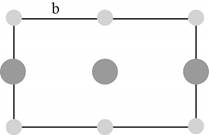
Figure 3
The lattice parameter
The relation between the length of the edge of a cube
The diagonal of the triangle shown in Figure (3) is
Substitute the value of
Therefore, the closest
The closest
(c)
Interpretation:
The closest
Concept introduction:
A unit cell of the crystal is the three-dimensional arrangement of the atoms present in the crystal. The unit cell is the smallest and simplest unit of the crystal which on repetition forms an entire crystal. Unit cell can be a cubic unit cell or hexagonal unit cell. The classification of a unit cell depends on the lattice site occupied by the atoms.
Answer to Problem 22.35E
The closest
Explanation of Solution
The structure of a face-centered cubic lattice is shown below.
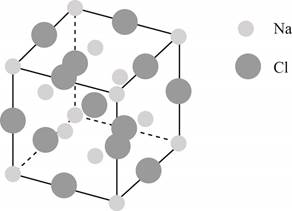
Figure 1
The plane of face-centered cubic lattice that has
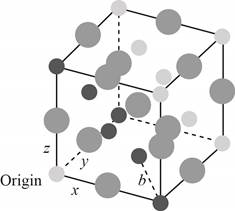
Figure 4
The side of the trigonal plane is same as the diagonal of the of the cube.
The lattice parameter
The relation between the length of the edge of a cube
The diagonal of the triangle shown in Figure (4) is
Substitute the value of
Therefore, the closest
The closest
Want to see more full solutions like this?
Chapter 22 Solutions
Bundle: Physical Chemistry, 2nd + Student Solutions Manual
- give the products for each of the followingarrow_forwardProvide the products and/or reagents for the following transformations. NaOMe HCl/EtOH OH NaOMe CI Show the product for the formation of the ketal given below for the transformation, showing all intermediates and resonance structures would be required to transform the ketal back to the starting ketone and then the mechanism What reagents/conditions HCI EtOH (excess)arrow_forwardMake meta-dibromobenze from nitrobenzene using amine reactions. *see imagearrow_forward
- Provide the structure of the expected major and minor (if any) products for each reaction. Clearly indicate stereochemistry where warranted. + + heat heat 이요 HNO3 1. AlCl3 2. H₂O H2SO4 1. AlCl3arrow_forward) Give the mechanism for the acid catalyzed hydrolysis of the following to the corresponding carboxylic acid. Show all intermediates and resonance structures N H+, H2O (excess)arrow_forward# 2. Drow full structures of the organic product expected in each of the following reactions. Draw the appropriate stereoisomer where warranted! Tos Cl O C NaCN PCC శ్రీ CI TSCI Pyridine H₂CrO4 PBrj Pyridine NaCNarrow_forward
- PLEASE help. Locate a literature IR spectrum of eugenol. Insert the literature spectrum here: What conclusions can you draw about your clove oil from these IR spectra? I attached my data belowarrow_forwardplease help and the percent recovery of clove oil from cloves is 4.61% and i have attached my ir spectrum as well. Based on your GC data, how many components are in the clove oil? Calculate the percentage of each component. Clearly show your work. Which of the components corresponds to eugenol? How do you know? Is eugenol the major component?arrow_forwardplease help and i am so confused if the picture is the gc data or ir spectrum. you dont have to do everything just what you can please because i am lost and the mass of the cloves was Mass of cloves 62.299g. Mass of recovered clove oil 62.761g.arrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





