
(a)
Interpretation:
In the given reaction, the
Concept introduction:
A Friedel-Craft’s alkylation reaction does not occur readily unless the halogen atom of the
Answer to Problem 22.26P
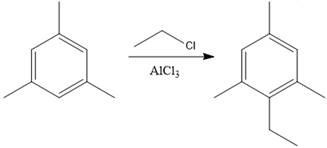
The product of the given Friedel-Craft’s alkylation reaction is as shown:
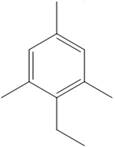
Explanation of Solution
The given reaction is Friedal Craft’s alkylation reaction. In the first step,
With the help of mechanism of electrophilic aromatic substitution reaction, the product of the given Friedel-Craft’s alkylation reaction was determined.
(b)
Interpretation:
In the given reaction, the aromatic ring has just one chemically distinct, aromatic H, so a single electrophilic aromatic substitution will lead to just a single product. The product of the given reaction is to be determined.
Concept introduction:
Electrophiles in electrophilic aromatic substation reactions typically must be generated in situ from more stable precursors that can be added as starting materials. Aluminum chloride acts as a Lewis acid and forms a complex with chlorine. After heterolysis, electrophilic aromatic substitution reaction takes place. The arenium ion intermediate is a carbocation intermediate consisting of five
Answer to Problem 22.26P
The product of the given halogenation reaction is as shown:
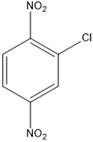
Explanation of Solution
The given reaction is Friedal Craft’s alkylation reaction. In the first step
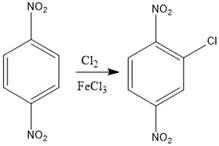
With the help of mechanism of electrophilic aromatic substitution reaction, the product of the given reaction was determined.
(c)
Interpretation:
In the given reaction, the aromatic ring has just one chemically distinct, aromatic H, so a single electrophilic aromatic substitution will lead to a just a single product. The product of the given reaction is to be determined.
Concept introduction:
In Friedel Craft’s acylation reaction, the aromatic species is treated with acyl chloride. The product of Friedel Craft’s acylation reaction is
Answer to Problem 22.26P
The product of the given Friedel-Craft’s acylation reaction is as shown.
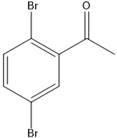
Explanation of Solution
The given reaction is Friedal Craft’s acylation reaction. In the first step,
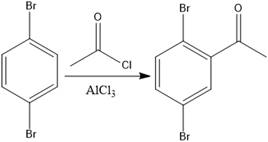
With the help of the mechanism of electrophilic aromatic substitution reaction, the product of the given Friedel-Craft’s acylation reaction was determined.
Want to see more full solutions like this?
Chapter 22 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- How many hydrogen atoms are connected to the indicated carbon atom?arrow_forwardIdentify the compound with the longest carbon - nitrogen bond. O CH3CH2CH=NH O CH3CH2NH2 CH3CH2C=N CH3CH=NCH 3 The length of all the carbon-nitrogen bonds are the samearrow_forwardIdentify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forward
- H H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forwardRank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forwardRank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forward
- choose the description that best describes the geometry for the following charged species ch3-arrow_forwardWhy isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forwardWhat is the approximate bond angle around the nitrogen atom? HNH H Harrow_forward
- OH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forwardComplete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forwardQ Select to Edit NH3 (CH3)2CHCI (1 equiv) AICI 3 Select to Draw cat. H2SO4 SO3 (1 equiv) HO SOCl2 pyridine Select to Edit >arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





