
EBK CHEMISTRY: THE MOLECULAR NATURE OF
7th Edition
ISBN: 9781119513216
Author: HYSLOP
Publisher: JOHN WILEY+SONS INC.
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 22, Problem 102RQ
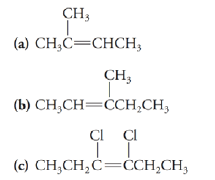
Write the structures of the cis and tram isomers, if any, for the following compounds:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Define the term “transition.” How does this definition apply to the transition metals?
Describe how the properties of the different types of elements (metals, nonmetals, metalloids) differ.
Use a textbook or other valid source to research the physical and chemical properties of each element listed in Data Table 1 using the following as a guideline:
Ductile (able to be deformed without losing toughness) and malleable (able to be hammered or pressed permanently out of shape without breaking or cracking) or not ductile or malleable
Good, semi, or poor conductors of electricity and heat
High or low melting and boiling points
Occur or do not occur uncombined/freely in nature
High, intermediate, or low reactivity
Loses or gains electrons during reactions or is not reactive
Chapter 22 Solutions
EBK CHEMISTRY: THE MOLECULAR NATURE OF
Ch. 22 - Prob. 1PECh. 22 - Prob. 2PECh. 22 - Prob. 3PECh. 22 - Practice Exercise 22.4
Write the IUPAC names of...Ch. 22 - Oxidation of an alcohol gave the following...Ch. 22 - Prob. 6PECh. 22 - Prob. 7PECh. 22 - Prob. 8PECh. 22 - Prob. 9PECh. 22 - Prob. 10PE
Ch. 22 - Complete the following equation by drawing...Ch. 22 - Prob. 12PECh. 22 - Prob. 13PECh. 22 - Prob. 14PECh. 22 - Prob. 15PECh. 22 - Label the hydrophobic and hydrophilic portions of...Ch. 22 - Draw a ribose ring and a deoxyribose ring. What is...Ch. 22 - Practice Exercise 22.18
Which base pairs match in...Ch. 22 - Prob. 1RQCh. 22 - Prob. 2RQCh. 22 - Prob. 3RQCh. 22 - Prob. 4RQCh. 22 - Prob. 5RQCh. 22 - Prob. 6RQCh. 22 - Prob. 7RQCh. 22 - Which of the following compounds has the higher...Ch. 22 - In general terms, why do functional groups impart...Ch. 22 - Prob. 10RQCh. 22 - What is the difference between geometric isomers...Ch. 22 - Prob. 12RQCh. 22 - No number is needed to identify the location of...Ch. 22 - Prob. 14RQCh. 22 - Prob. 15RQCh. 22 - Prob. 16RQCh. 22 - 22.17 In general terms, why doesn't benzene...Ch. 22 - Prob. 18RQCh. 22 - 22.19 Explain why is more soluble in water than ....Ch. 22 - Prob. 20RQCh. 22 - Prob. 21RQCh. 22 - Why do aldehydes and ketones have boiling points...Ch. 22 - Acetic acid boils at 118C, higher even than...Ch. 22 - Methyl ethanoate has many more atoms than its...Ch. 22 - Prob. 25RQCh. 22 - 22.26 Write condensed structures of the following...Ch. 22 - 3-Butanol is not a proper name, but a structure...Ch. 22 - Prob. 28RQCh. 22 - Prob. 29RQCh. 22 - Prob. 30RQCh. 22 - Prob. 31RQCh. 22 - Prob. 32RQCh. 22 - Amines, RNH2, do not have boiling points as high...Ch. 22 - A monofunctional organic nitrogen compound...Ch. 22 - Prob. 35RQCh. 22 - Prob. 36RQCh. 22 - Prob. 37RQCh. 22 - Write the products that can be expected to form in...Ch. 22 - Prob. 39RQCh. 22 - Prob. 40RQCh. 22 - 22.41 What do we mean by the term polymer...Ch. 22 - Prob. 42RQCh. 22 - Prob. 43RQCh. 22 - Prob. 44RQCh. 22 - Prob. 45RQCh. 22 - Prob. 46RQCh. 22 - Prob. 47RQCh. 22 - Prob. 48RQCh. 22 - Prob. 49RQCh. 22 - Prob. 50RQCh. 22 - Prob. 51RQCh. 22 - Prob. 52RQCh. 22 - Prob. 53RQCh. 22 - Prob. 54RQCh. 22 - Prob. 55RQCh. 22 - Prob. 56RQCh. 22 - Prob. 57RQCh. 22 - Prob. 58RQCh. 22 - What are the three fundamental needs for...Ch. 22 - Prob. 60RQCh. 22 - Prob. 61RQCh. 22 - Prob. 62RQCh. 22 - Name the compounds that form when sucrose is...Ch. 22 - Prob. 64RQCh. 22 - Prob. 65RQCh. 22 - Prob. 66RQCh. 22 - Prob. 67RQCh. 22 - 22.68 What function is served by glycogen in the...Ch. 22 - How are lipids defined?Ch. 22 - Why are lipids more soluble than carbohydrates in...Ch. 22 - 22.71 Cholesterol is not an ester, yet it is...Ch. 22 - A product such as corn oil is advertised as...Ch. 22 - Is it likely that the following compound could be...Ch. 22 - Describe the specific ways in which the monomers...Ch. 22 - What is the peptide bond? How is it similar to the...Ch. 22 - Prob. 76RQCh. 22 - Prob. 77RQCh. 22 - Why is a distinction made between the terms...Ch. 22 - Prob. 79RQCh. 22 - What kind of substance makes up most enzymes?Ch. 22 - Prob. 81RQCh. 22 - Prob. 82RQCh. 22 - Prob. 83RQCh. 22 - 22.84 How are the two DNA strands in a double...Ch. 22 - In what ways do DNA and RNA differ structurally.Ch. 22 - 22.86 Which base pairs with
Ch. 22 - The process of transcription begins with which...Ch. 22 - The process of translation begins with which...Ch. 22 - Prob. 89RQCh. 22 - 22.90 Write full (expanded) structures for each of...Ch. 22 - Prob. 91RQCh. 22 - Prob. 92RQCh. 22 - Prob. 93RQCh. 22 - Prob. 94RQCh. 22 - Prob. 95RQCh. 22 - Prob. 96RQCh. 22 - Prob. 97RQCh. 22 - Prob. 98RQCh. 22 - Prob. 99RQCh. 22 - Prob. 100RQCh. 22 - Prob. 101RQCh. 22 - 22.102 Write the structures of the cis and tram...Ch. 22 - 22.103 Write the structures of the products that...Ch. 22 - Prob. 104RQCh. 22 - 22.105 Repeat Problem 22.103 using...Ch. 22 - Repeat Problem 22.104 using cyclohexene. The...Ch. 22 - Prob. 107RQCh. 22 - Predict the products of the reaction of benzene...Ch. 22 - Prob. 109RQCh. 22 - Prob. 110RQCh. 22 - Prob. 111RQCh. 22 - Prob. 112RQCh. 22 - Prob. 113RQCh. 22 - Write the structure of the product of the...Ch. 22 - Prob. 115RQCh. 22 - Prob. 116RQCh. 22 - Prob. 117RQCh. 22 - Prob. 118RQCh. 22 - Prob. 119RQCh. 22 - Prob. 120RQCh. 22 - Prob. 121RQCh. 22 - Write the structures of the products that form in...Ch. 22 - Prob. 123RQCh. 22 - Prob. 124RQCh. 22 - Prob. 125RQCh. 22 - Prob. 126RQCh. 22 - Prob. 127RQCh. 22 - Prob. 128RQCh. 22 - 22.129 Write the structure of a triacylglycerol...Ch. 22 - 22.130 Write the structures of the products of the...Ch. 22 - Write the structure of the triacylglycerol that...Ch. 22 - *22.132 If the compound in Problem 22.130 is...Ch. 22 - *22.133 What parts of glyccrophospholipid...Ch. 22 - *22.134 In general terms, describe the structure...Ch. 22 - Prob. 135RQCh. 22 - What is the structure of the tripeptide that could...Ch. 22 - 22.137 What are the structures of the two...Ch. 22 - Prob. 138RQCh. 22 - Prob. 139RQCh. 22 - Prob. 140RQCh. 22 - Prob. 141RQCh. 22 - 22.142 Suggest a reason why trimethylamine, , has...Ch. 22 - Prob. 143RQCh. 22 - How many tripeptides can be made from three...Ch. 22 - Prob. 145RQCh. 22 - Prob. 146RQCh. 22 - Estimate the number of kilojoules of heat that...Ch. 22 - Prob. 148RQCh. 22 - Prob. 149RQCh. 22 - The compound that causes your eyes to water when...Ch. 22 - Prob. 151RQCh. 22 - Prob. 152RQCh. 22 - Use resonance structures to explain why urea,...Ch. 22 - Prob. 154RQCh. 22 - Prob. 155RQ
Additional Science Textbook Solutions
Find more solutions based on key concepts
6.1 State the number of electrons that be must be lost by atoms of each of the following to achieve a stable el...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
What dipeptides would be formed by heating a mixture of valine and N-protected leucine?
Organic Chemistry (8th Edition)
Classify each pure substance as an element or a compound. a. aluminum b. sulfur c. methane d. acetone
Introductory Chemistry (6th Edition)
What type of cut would separate the brain into anterior and posterior parts?
Anatomy & Physiology (6th Edition)
A 1500 kg car is rolling at 2.0 m/s. You would like to stop the car by firing a 10 kg blob of sticky clay at it...
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
39. What are the units of k for each type of reaction?
a. first-order reaction
b. second-order reaction
c...
Chemistry: Structure and Properties (2nd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Provide the Physical and Chemical Properties of Elements of the following elements listedarrow_forwardQuestions 4 and 5arrow_forwardFor a titration of 40.00 mL of 0.0500 M oxalic acid H2C2O4 with 0.1000 M KOH, calculate the pH at each of the following volume of KOH used in the titration: 1) before the titration begin;2) 15 mL; 3) 20 mL; 4) 25 mL; 5) 40 mL; 6) 50 mL. Ka1 = 5.90×10^-2, Ka2 = 6.50×10^-5 for oxalic acid.arrow_forward
- Predict the major organic product(s), if any, of the following reactions. Assume all reagents are in excess unless otherwise indicated.arrow_forwardPredict the major organic product(s), if any, of the following reactions. Assume all reagents are in excess unless otherwise indicated.arrow_forwardHow many signals would you expect to find in the 1 H NMR spectrum of each given compound? Part 1 of 2 2 Part 2 of 2 HO 5 ☑ Х IIIIII***** §arrow_forward
- A carbonyl compound has a molecular ion with a m/z of 86. The mass spectra of this compound also has a base peak with a m/z of 57. Draw the correct structure of this molecule. Drawingarrow_forwardCan you draw this using Lewis dot structures and full structures in the same way they are so that I can better visualize them and then determine resonance?arrow_forwardSynthesize the following compound from cyclohexanol, ethanol, and any other needed reagentsarrow_forward
- For a titration of 20.00 mL of 0.0500 M H2SO4 with 0.100 M KOH, calculate the pH at each of the following volume of KOH used in the titration: 1) before the titration begin; 2) 10.00 mL; 3) 20.00 mL; 4) 30.00 mL. Ka2 = 1.20×10-2 for H2SO4.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s) Be sure to account for all bond-breaking and bond-making steps Problem 73 of 10 Drawing Amows ro HO Donearrow_forward12. Synthesize the following target molecules (TMs) using the specified starting materials. .CI a) HO3S SM TM b) HO- SMarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY