
Concept explainers
Predict the product(s) and provide the mechanism for each reaction below.
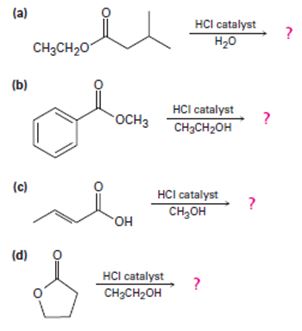
a)
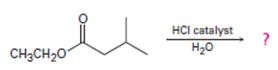
Interpretation:
Including from the reactants, represents and the products, the reaction is essentially, Hydraysis of Ester by acid catalysis involving the AAc2 mechansim.
Ester are hydrolyzed with water in the presence of either of acid or base, to form carboxylic acids or carboxylate anians respective, and the corresponding alcohol.
Concept introduction:
Carboxylic acids react with alcohols to form esters through a condensation reaction known as Esterification.
Hydrolysis is base or acid–catalysed, and esterification is only acid catalyzed.
Eight mechanism are possible for ester hydrolysis, but only 6 have been observed. Since in principle, the acid catalyzed reaction (hydrolysis and esterification) are reversible, but not alkaline hydrolysis, four mechanism are possible for esterification. Among only three have been observed.
| Designation | Explanation | Hydrolysis | Esterification |
|---|---|---|---|
| BAC1 | Unimolecular (1) base catalyzed by acyl oxygen(AC) cleavage | X | X |
| BAC2 | Base catalyzed (B) bimolecular (2) by Acyl-oxygen cleavage. | Very common | X |
| AAC1 | Acid catalyzed (A) Unimolecular by Alkyl-oxygen cleavage. | Special cases | Special cases |
| AAc2 | Acid catalyzed bimolecular by Acyl-oxygen cleavage. | Very common | Very common |
| BAC1 | Base catalyzed Unimolecular by Alkyl-oxygen cleavage. | Special cases | X |
| BAC2 | Base catalyzed bimolecular by Alkyl-oxygen cleavage. | rare | X |
| AAL1 | Acid catalyzed(A) Unimolecular by Alkyl-oxygen cleavage. | Common for tert alcohol | Common for tert alcohol |
| AAL2 | Acid catalyzed bimolecular by Alkyl-oxygen cleavage | X | X |
All four of the given reaction to the AAc2 type, Acid catalyzed bimolecular hydrolysis of the given ester by Acyl-oxygen cleavage.
This mechanism is detailed below in general -----(1)
The reaction for 21.33 (C) involves esterification of the given acide by the same AAc2 mechanism and is detailed in II below.
I AAc2 ester hydrolysis the most general mechanism, this is a bimolecular Reversible hydrolysis. Hence according to the principle of microcepic reversibility, the mechanism of acid catalyzed esterification is the exact revenge of that of acid catalyzed hydrolysis.
Reaction is essentially a nucleophilic acyl substribution (an addition-elimination process mediated by nucleophilic and leaving groups) at the acyl carbon.
Answer to Problem 33MP
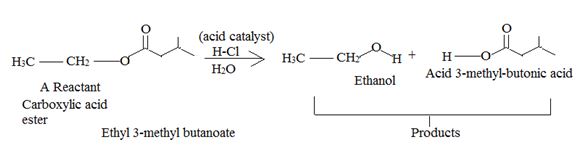
Explanation of Solution
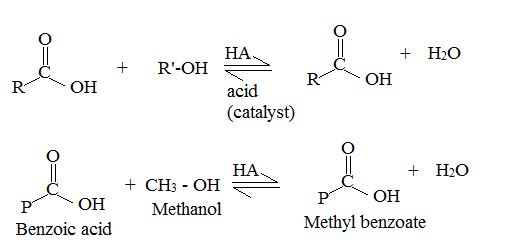
This type of acid catalysed Esterification is called fisher Esterification.
They proceed very slowly in the absence of strong acids. But they reach equilibrium within a few hours, when an acid and an alcohol are refluxed with a small amount of concentrated sulfuric acid (H2SO4) or hydrogen chloride (H-Cl), Since the position of equilibrium controls the amount of the ester formed, the use of an excess of either the carboxylic acids or the alcohol increases the yield based on the limiting reactant. The choice of component in excess depends on its availability. The Yield of an Esterification reaction can also be increased bt the remaining the water from the reaction mixture as it is formed.
Reaction 1 is an equilibrium, Reversible reaction.
Now we follow the forward reactions in the mechanism (to be explained), we have the mechanism for the acid catalyzed Esterification of an acid. If however we follow the reverse reaction. We have the mechanism for the acid catalyzed hydrolysis of an ester.
Acid catalyzed ester hydrolysis
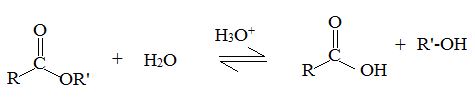
Which result we obtain will depends on the conditions we choose. If we want to esterify an acid, we use an excess of the alcohol and if possible, remove the water as it formed.
Now, we need to hydrolysis the ester, we use a large excess of water, i.e we reflux the ester with dilute aqueous HCl or dilute aqueous H2SO4.
A plurality of mechanism of hydrolysis of carboxylic esters and esterification of carboxylic acids are theoretically possible due to the following reactions.
Hydrolysis and esterification may proceed either through acyl oxygen bond cleavage of an alkyl oxygen bond cleavage.
Acyl-oxygen cleavage

Alkyl-oxygen cleavage

Thus acidic hydrolysis of the given ester has occurred by the Bimolecular AAc2 mechanism and the products are Ethanol and 3-methyl-1-butanoic acid.
b)
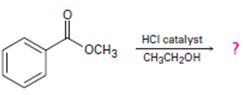
Interpretation:
Including from the reactants, represents and the products, the reaction is essentially, Hydraysis of Ester by acid catalysis involving the AAc2 mechansim.
Ester are hydrolyzed with water in the presence of either of acid or base, to form carboxylic acids or carboxylate anians respective, and the corresponding alcohol.
Concept introduction:
Carboxylic acids react with alcohols to form esters through a condensation reaction known as Esterification.
Intropection of the reactant (A) ester, the reagents and the produts (p) suggests that its is a Trans esterification process by anacid catalyzed bimolecular AAc2 mechanism.
Answer to Problem 33MP
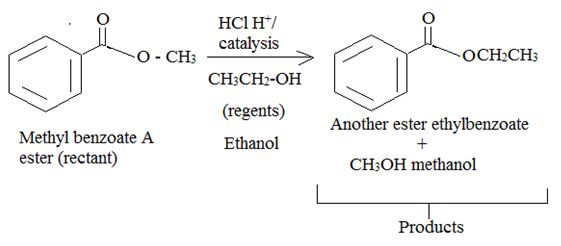
Explanation of Solution
Trans esterification is essentially the inter conversion of one ester to another also called alcoholysis, since the effective nucleophile is an alcohol, the reaction, can be catalysed by born on acid (H2SO4 or dry HCl) or base (usually alkoxide) it follows the same nucleophile addition elimination process at and acyl corbon.
Mechanism:
Acid catalysed AAc2 trans esterification by acyl-oxygen cleavase.
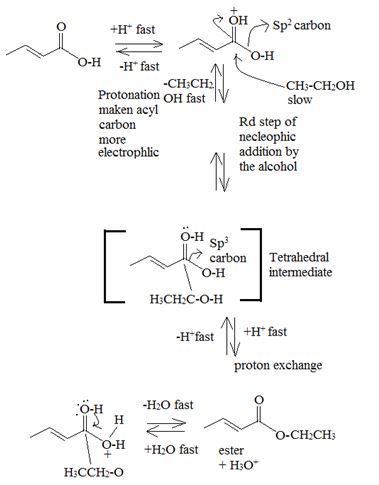
Since each step of the process is an equilibrium one and thermodynamically reversible the forward process is favoured by adding catalytic amount of cone H2SO4 to remove the water as soon as it is formed and increase the yield of the ester. Simultaneously, a laye exam of the ethanol must also be used.
Thus by trans esterification is the AAc2 mechanism the given ester has been converted to an other one.
Products or ethyl benzoate and water.
c)
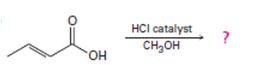
Interpretation:
Including from the reactants, represents and the products, the reaction is essentially, Hydraysis of Ester by acid catalysis involving the AAc2 mechansim.
Ester are hydrolyzed with water in the presence of either of acid or base, to form carboxylic acids or carboxylate anians respective, and the corresponding alcohol.
Concept introduction:
Carboxylic acids react with alcohols to form esters through a condensation reaction known as Esterification.
Answer to Problem 33MP
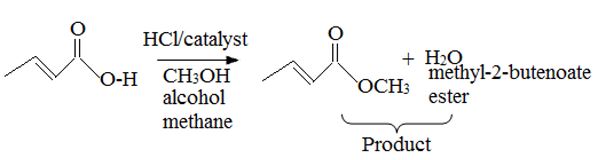
The given reactant is 2-butoni c acid has been converted to the ester-methyl-2-butoni c by AAc2 mechanism via the esterification. Adding c few drops of conc H2SO4 Removes the water formed and also known the catalysis.
Explanation of Solution
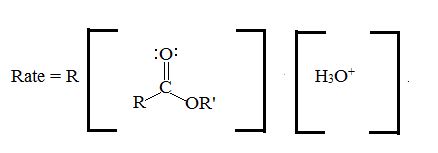
The catalytic effect of mineral acids like H2SO4, HCl, H3PO4 is to enhance the electrophili of the catbonic carbon by presentation of its oxygen, on the forward process, (hydrolysis) water acts as a nucleophilic on the acyl carbon, and displaces the alcohol. R1OH (good leaving group) to form an acid in the intermediate I and esterification I and II. On the backward process esterification, alcohol acts as a nucleophilic and displace water to form an ester via the same intermediate.
Kinetic studies conform the bimolecular nature
Evidence-Evidence for the acyl oxygen bond cleavage has been obtained from 18O Labeling experiments and steru chemical studies. For example, the acid catalyzed hydrolysis of methyl hydrogen successive in water enriched with 18O, Gives methanol with no extra 18O. Thus, Acyl-oxygen hydrolysis must have occurred.
3) Hydrolysis and esterification may proceed by unimolecular of a bimolecular mechanism.
c) On examing the reactant, reagents and the products, it follows that the reaction is esterification of the given acid, and not an ester hydrolysis.
Concept: Since, esterification of a carboxylic acid [ie synthesis of ester] and hydrolysis of an acid by acid catalysis, is a reversible equilibrium reaction the reverse reaction of the AAc2 mechanism is followed. A layer excess of the alcohol methanol in refluxed with acid in the presence of catalytic amount of HCl, and the product water fast removed from the system to ensure forward completion of the reaction. This is the scheme of Fischer esterification, and in effect a few dup of cone H2SO4 are added in place of HCl to have a catalytic effect and also remove the product water as sonar it is formed. The mechanism to AAc2 involving the Nucleophilic addition elimination process at the acyl carbon; The Nucleophilic this time is an alcohol, methanol.
The given reactant is 2-butoni c acid has been converted to the ester-methyl-2-butoni c by AAc2 mechanism via the esterification. Adding c few drops of conc H2SO4 Removes the water formed and also known the catalysis.
d)
Interpretation:
Including from the reactants, represents and the products, the reaction is essentially, Hydraysis of Ester by acid catalysis involving the AAc2 mechansim.
Ester are hydrolyzed with water in the presence of either of acid or base, to form carboxylic acids or carboxylate anians respective, and the corresponding alcohol.
Concept introduction:
Carboxylic acids react with alcohols to form esters through a condensation reaction known as Esterification.
Answer to Problem 33MP
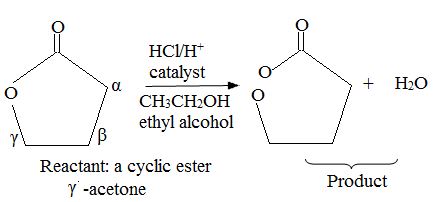
Explanation of Solution
Evidently, reaction is a trans esterification reaction of the r-lectone (reactant). Reaction mechanism, explained below is AAc2, ie Acid catalysed Bimolecular by acyl oxygen cleavage. A occurs by a new addition and elimination process at the acyl.
Lectones/lectams and thus cyclic ester can be similar converted by same nuclephilic acyl substitution reactions, except the acid is cleaved.
Want to see more full solutions like this?
Chapter 21 Solutions
Organic Chemistry - With Access (Custom)
- 19.78 Write the products of the following sequences of reactions. Refer to your reaction road- maps to see how the combined reactions allow you to "navigate" between the different functional groups. Note that you will need your old Chapters 6-11 and Chapters 15-18 roadmaps along with your new Chapter 19 roadmap for these. (a) 1. BHS 2. H₂O₂ 3. H₂CrO4 4. SOCI₂ (b) 1. Cl₂/hv 2. KOLBU 3. H₂O, catalytic H₂SO4 4. H₂CrO4 Reaction Roadmap An alkene 5. EtOH 6.0.5 Equiv. NaOEt/EtOH 7. Mild H₂O An alkane 1.0 2. (CH3)₂S 3. H₂CrO (d) (c) 4. Excess EtOH, catalytic H₂SO OH 4. Mild H₂O* 5.0.5 Equiv. NaOEt/EtOH An alkene 6. Mild H₂O* A carboxylic acid 7. Mild H₂O* 1. SOC₁₂ 2. EtOH 3.0.5 Equiv. NaOEt/E:OH 5.1.0 Equiv. NaOEt 6. NH₂ (e) 1. 0.5 Equiv. NaOEt/EtOH 2. Mild H₂O* Br (f) i H An aldehyde 1. Catalytic NaOE/EtOH 2. H₂O*, heat 3. (CH,CH₂)₂Culi 4. Mild H₂O* 5.1.0 Equiv. LDA Br An ester 4. NaOH, H₂O 5. Mild H₂O* 6. Heat 7. MgBr 8. Mild H₂O* 7. Mild H₂O+arrow_forwardLi+ is a hard acid. With this in mind, which if the following compounds should be most soluble in water? Group of answer choices LiBr LiI LiF LiClarrow_forwardQ4: Write organic product(s) of the following reactions and show the curved-arrow mechanism of the reactions. Br MeOH OSO2CH3 MeOHarrow_forward
- Provide the correct IUPAC name for the compound shown here. Reset cis- 5- trans- ☑ 4-6- 2- 1- 3- di iso tert- tri cyclo sec- oct but hept prop hex pent yl yne ene anearrow_forwardQ6: Predict the major product(s) for the following reactions. Note the mechanism (SN1, SN2, E1 or E2) the reaction proceeds through. If no reaction takes place, indicate why. Pay attention to stereochemistry. NaCN DMF Br σ Ilm... Br H Br H H NaCN CH3OH KOtBu tBuOH NaBr H₂O LDA Et2O (CH3)2CHOH KCN DMSO NaOH H₂O, A LDA LDA Systemarrow_forwardQ7: For the following reactions, indicate the reaction conditions that would provide the indicated product in a high yield. Note the major reaction pathway that would take place (SN1, SN2, E1, or E2) Note: There may be other products that are not shown. There maybe more than one plausible pathway. Br H3C OH H3C CI ... H3C SCH2CH3 CI i SCH2CH3 ཨ་ Br System Settarrow_forward
- Q2: Rank the compounds in each of the following groups in order of decreasing rate of solvolysis in aqueous acetone. OSO2CF3 OSO2CH3 OH a. b. CI Brarrow_forwardох 4-tert-butyl oxy cyclohex-1-ene Incorrect, 1 attempt remaining The systematic name of this compound classifies the -OR group as a substituent of the hydrocarbon, which is considered the principal functional group. The ether substituent is named with the suffix 'oxy'. The general format for the systematic name of a hydrocarbon is: [prefix/substituent] + [parent] + [functional group suffix] Substituents are listed in alphabetical order. Molecules with a chiral center will indicate the absolute configuration at the beginning of its name with the R and S notation.arrow_forward5. Compressibility (6 points total). The isothermal compressibility is a measure of how hard/easy it is to compress an object (how squishy is it?) at constant temperature. It is др defined as Br=-()=-(200²)T' (a) You might wonder why there is a negative sign in this formula. What does it mean when this quantity is positive and what does it mean when this quantity is negative? (b) Derive the formula for the isothermal compressibility of an ideal gas (it is very simple!) (c) Explain under what conditions for the ideal gas the compressibility is higher or lower, and why that makes sense.arrow_forward
- 19. (3 pts) in Chapter 7 we will see a reaction of halocyclohexanes that requires that the halogen occupy an axial position with this in mind, would you expect cis-1-bromo-3-methylcyclohexane or trans-1-bromo-3-methylcyclohexane to be more reactive in this reaction? Briefly explain your choice using structures to support your answer. Mere-eries-cecleone) The tran-i-browse-3-methylcyclohexionearrow_forwardPlease help me calculate the undiluted samples ppm concentration. My calculations were 280.11 ppm. Please see if I did my math correctly using the following standard curve. Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/EVSJL_W0qrxMkUjK2J3xMUEBHDu0UM1vPKQ-bc9HTcYXDQ?e=hVuPC4arrow_forwardProvide an IUPAC name for each of the compounds shown. (Specify (E)/(Z) stereochemistry, if relevant, for straight chain alkenes only. Pay attention to commas, dashes, etc.) H₁₂C C(CH3)3 C=C H3C CH3 CH3CH2CH CI CH3 Submit Answer Retry Entire Group 2 more group attempts remaining Previous Nextarrow_forward

 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



