
Connect Online Access 1-Semester for Organic Chemistry
6th Edition
ISBN: 9781260475609
Author: SMITH, Janice
Publisher: Mcgraw-hill Higher Education (us)
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21.8, Problem 19P
How can pentan-2-one be converted into each compound?
a. 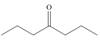 b.
b. 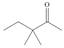 c.
c. 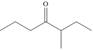 d.
d. 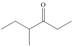
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
29.
a) i
Which energy diagram best represents the d-electrons in tetrahedral [Co(NH3)4]²+?
b) ii
c) iii
d) iv
11
་
↑↓ ↑t t
↑↓ ↑↓
e) none of these
ii
In1
According to Slater's rules, what is the effective nuclear charge experienced by a 3d electron in
30.
Ge?
a) 32.00
b) 21.15
c) 16.05
d) 14.00
e) 10.85
Regarding Lowis structuros and geometrios,
Draw Lewis structures for the following: SOF4, SO, ICI, XeO2F4, SeF and XeO3.
For each one, indicate the observed molecular geometry it adopts.
Explain the following statements with equations:
- The fusion product of an organic compund with sodium metal is an alkaline solution
- The test for elements should be done before the solubility tests.
- Using less sodium than the organic compound in the ignition test might cause a problem to detect the presence of the nitrogen and sulfur
- Formation of colored product when adding ferric acid chloride to phenol solution
Chapter 21 Solutions
Connect Online Access 1-Semester for Organic Chemistry
Ch. 21.2 - Problem 23.1 Draw the enol or keto tautomer(s) of...Ch. 21.2 - Problem 23.3 When phenylacetaldehyde is dissolved...Ch. 21.3 - Prob. 5PCh. 21.3 - Problem 23.5 Which bonds in the following...Ch. 21.3 - Prob. 7PCh. 21.3 - Prob. 8PCh. 21.3 - Prob. 9PCh. 21.4 - Prob. 10PCh. 21.5 - Prob. 11PCh. 21.7 - Problem 23.11 Draw the products of each...
Ch. 21.7 - Problem 23.12 Draw the products of each reaction....Ch. 21.7 - Prob. 14PCh. 21.7 - Prob. 15PCh. 21.8 - Prob. 16PCh. 21.8 - Prob. 17PCh. 21.8 - Prob. 18PCh. 21.8 - Problem 23.18 How can pentan-2-one be converted...Ch. 21.8 - Problem 23.19 Identify A, B, and C, intermediates...Ch. 21.9 - Problem 23.20 Which of the following compounds...Ch. 21.9 - Problem 23.21 Draw the products of each...Ch. 21.9 - Prob. 23PCh. 21.9 - Prob. 24PCh. 21.9 - Prob. 25PCh. 21.10 - Prob. 26PCh. 21.10 - Prob. 27PCh. 21.10 - Prob. 28PCh. 21.10 - Prob. 29PCh. 21 - 23.29 Draw enol tautomer(s) for each compound....Ch. 21 - 22.30 The cis ketone A is isomerized to a trans...Ch. 21 - 23.31 Draw enol tautomer(s) for each compound.
...Ch. 21 - Prob. 33PCh. 21 - Prob. 34PCh. 21 - 23.35 Rank the labeled protons in each compound in...Ch. 21 - Prob. 36PCh. 21 - Prob. 37PCh. 21 - 23.38 Acyclovir is an effective antiviral agent...Ch. 21 - 23.39 Explain why forms two different alkylation...Ch. 21 - Prob. 40PCh. 21 - 23.42 Draw a stepwise mechanism for the following...Ch. 21 - Prob. 42PCh. 21 - Prob. 43PCh. 21 - 23.45 Devise a synthesis of valproic acid , a...Ch. 21 - Prob. 57PCh. 21 - 23.57 Draw a stepwise mechanism showing how two...Ch. 21 - 23.58 Draw a stepwise mechanism for the following...Ch. 21 - Prob. 65PCh. 21 - 23.66 Synthesize (Z)-hept-5-en-2-one from ethyl...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 31 Indicate the symbol, mass number and the atomic number of the missing product in each of the following nuclear reactions. a) 13/3 N 41 b) 11 Ca 20 c) 90 38 Sr → 133 C + ? + - 6 0 e →? 90 Y + ? 39 11 d) 22 Na → ? + + 1 B +1 β Toarrow_forwardPlease drawarrow_forward9. compore the Following two Venctions IN termy Of Ronction Rate and explan in detail the reasoning that led to your conclusion +He p₁₂ 11- ㅐ 15 .. +He H #H H / H b. Compare the Following too reactions 14 terms of reaction Rate and explain in detail the reasoning that led to your conclusion Н d-C- tłu Na +2446 е -ll +2n "Harrow_forward
- a. •Write all of the possible products For the Following ronction А ----- H - H H + H₂0 H+ Н b. in Rite the complete reaction Mechaniszn For the Formation of each product. ·C. Suggest what Reaction conditions could Result in each product being the major Product of the veaction:arrow_forwarda. Write the product For each of the Following reactions H 6-836-6 레 +H₂ N A H A-C-C=C-C-CH + 2 Na +2 NH3 - H H b. Write the reaction Mechanism For. reaction eacharrow_forwardhelp draw the moleculearrow_forward
- How to draw this claisen condensation reaction mechanisms/arrow_forwardWrite all of Me Possible Products For each Of the Following reactions. In each case identity all pains of enantiomers, all digsterzoners and all Meso compounds 9. 11-60 11-0-11 V-G Η Η H ~ C-11 +HB+ - 1 H b. पन्ना 171-0-11 H-C-H Н C-C=c-call +HBr Perendez ==arrow_forwardHow can i draw the mechanisms for this molecule?arrow_forward
- a. Discuss and explain he difference IN Stability between the Chai and Boat Гольцу от судомехане b. For the Following Molecule draw both possible Clain conformations and explain which one is more stable and for what Reason. H. CH₂ CH₂ H "Harrow_forwarddraw out these molecules pleasearrow_forwardhelp draw any straightchain moleculearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY