
(a)
Interpretation:
To identify the major product in the given set of reactions when treated with
Concept introduction:
The reaction between lithium dialkyl cuprate (Michael donor) which acts as a nucleophile for 1,4-conjugate addition to
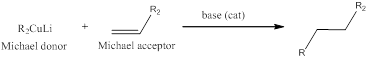
The formed enolate when treated with
(b)
Interpretation:
To identify the major product in the given set of reactions when treated with
Concept introduction:
The reaction between lithium dialkyl cuprate (Michael donor) which acts as a nucleophile for 1,4-conjugate addition to
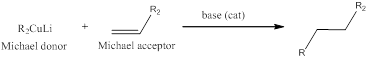
The formed enolate when treated with alkyl halide installs the alkyl group in the
Reduction of
(c)
Interpretation:
To identify the major product in the given set of reactions when treated with
Concept introduction:
The reaction between lithium dialkyl cuprate (Michael donor) which acts as a nucleophile for 1,4-conjugate addition to
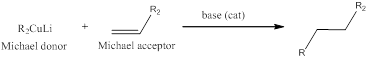
The formed enolate when treated with alkyl halide installs the alkyl group in the
Oxidation of the aldehyde with chromic acid gives the respective
(d)
Interpretation:
To identify the major product in the given set of reactions when treated with
Concept introduction:
Hydrolysis of acetal with aqueous acid leads to ketone.
The reaction between lithium dialkyl cuprate (Michael donor) which acts as a nucleophile for 1,4-conjugate addition to
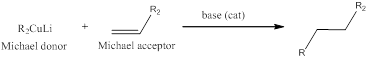
The formed enolate when treated with alkyl halide installs the alkyl group in the
When ketone is treated with ethylene glycol, the keto group is converted into an acetal.
(e)
Interpretation:
To identify the major product in the given set of reactions when treated with
Concept introduction:
Alcohol when treated with
The reaction between lithium dialkyl cuprate (Michael donor) which acts as a nucleophile for 1,4-conjugate addition to
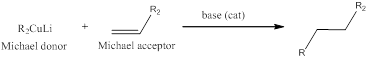
The formed enolate when treated with alkyl halide installs the alkyl group in the
Imine is formed when aldehyde is treated with a primary
(f)
Interpretation:
To identify the major product in the given set of reactions when treated with
Concept introduction:
Alcohol when treated with
The reaction between lithium dialkyl cuprate (Michael donor) which acts as a nucleophile for 1,4-conjugate addition to
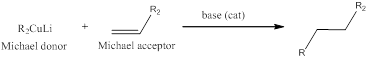
The formed enolate when treated with alkyl halide installs the alkyl group in the
Clemmenson reduction of aldehyde gives
Want to see the full answer?
Check out a sample textbook solution
Chapter 21 Solutions
Organic Chemistry Third Edition + Electronic Solutions Manual And Study Guide
- In the drawing areas below, draw the two most expected stable conformations of the following molecule: ייון Be sure your drawings make it possible to distinguish between the conformations. After you've drawn the conformations, answer the question below the drawing areas. Х S : ☐ ☑ 5arrow_forwardDraw the structure of the organic reactant, and write the chemical formula of the reagent used to form the given product. Click and drag to start drawing a structure. + T ☑ OH NO₂ NO2arrow_forwardNonearrow_forward
- Safari File Edit View History Bookmarks Window Help く < mylabmastering.pearson.com Wed Feb 12 8:44 PM ✩ + Apple Q Bing Google SignOutOptions M Question 36 - Lab HW BI... P Pearson MyLab and Mast... P Course Home Error | bartleby b Answered: If the biosynth... Draw a free-radical mechanism for the following reaction, forming the major monobromination product: ScreenPal - 2022 CHEM2... Access Pearson 2 CH3 Br-Br CH H3 Draw all missing reactants and/or products in the appropriate boxes by placing atoms on the canvas and connecting them with bonds. Add charges where needed. Electron- flow arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created. Include all free radicals by right-clicking on an atom on the canvas and then using the Atom properties to select the monovalent radical. ▸ View Available Hint(s) 0 2 DE [1] H EXP. CONT. H. Br-Br H FEB 12arrow_forwardPlease correct answer and don't use hand ratingarrow_forwardNonearrow_forwardQ1: For each molecule, assign each stereocenter as R or S. Circle the meso compounds. Label each compound as chiral or achiral. + CI Br : Н OH H wo་ཡིག་ཐrow HO 3 D ။။ဂ CI Br H, CI Br Br H₂N OMe R IN I I N S H Br ជ័យ CI CI D OHarrow_forwardPlease correct answer and don't use hand ratingarrow_forwardNonearrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





