
CHEM 262 ORG CHEM EBOOK DIGITAL DELIVERY
8th Edition
ISBN: 2818440043505
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2.10, Problem 43P
A naturally occurring amino acid such as alanine has a group that is a
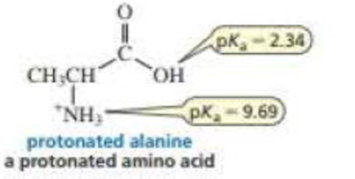
- a. If the pKa value of a carboxylic add such as acetic acid is about 5 (see Table 2.1 ), then why is the pKa value of the carboxylic acid group of alanine so much lower?
- b. Draw the structure of alanine in a solution at pH = 0.
- c. Draw the structure of alanine in a solution at physiological pH (pH 7.4).
- d. Draw the structure of alanine in a solution at pH = 12.
- e. Is there a pH at which alanine is uncharged (that is. neither group has a charge)?
- f. At what pH does alanine have no net charge (that is, the amount of negative charge is the same as the amount of positive charge)?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
(III) adsorbent
(b) Adsorption of the hexacyanoferrate (III) ion, [Fe(CN)6] ³, on y-Al2O3 from aqueous
solution was examined. The adsorption was modelled using a modified Langmuir
isotherm, yielding the following values of Kat pH = 6.5:
(ii)
T/K
10-10 K
280
2.505
295
1.819
310
1.364
325
1.050
Determine the enthalpy of adsorption, AadsHⓇ.
If the reported value of entropy of adsorption, Aads Se = 146 J K-1 mol-1 under the above
conditions, determine Aads Gº.
with full details solution please
write IUPAC names for these alcohols
Chapter 2 Solutions
CHEM 262 ORG CHEM EBOOK DIGITAL DELIVERY
Ch. 2.1 - Which of the following are not acids? CH3COOH CO2...Ch. 2.1 - Consider the following reaction: a. What is the...Ch. 2.1 - Draw the products of the addbase renc1 ion when a....Ch. 2.1 - a. What is the conjugate acid of each or the...Ch. 2.2 - a. Which is a stronger acid: one with a pKa of 5.2...Ch. 2.2 - An acid has a Ka of 4.53 106 in water. What is...Ch. 2.2 - Prob. 7PCh. 2.2 - Antacids are compounds that neutralize stomach...Ch. 2.2 - Are the following body fluids acidic or basic? a....Ch. 2.3 - Draw the conjugate acid of each of the following:...
Ch. 2.3 - a. Write an equation showing CH3OH reacting as an...Ch. 2.3 - Estimate the pKa values of the following...Ch. 2.3 - a. Which is a stronger base: CH3COO or HCOO? (The...Ch. 2.3 - Using the pKa values in Section 2.3, rank the...Ch. 2.4 - Prob. 15PCh. 2.5 - a. For each of the acid-base reactions in Section...Ch. 2.5 - Ethyne has a pKa value of 25, water has a pKa...Ch. 2.5 - Which of the following bases can remove a proton...Ch. 2.5 - Calculate the equilibrium constant for the...Ch. 2.6 - Rank the ions (CH3, NH2, HO, and F) from most...Ch. 2.6 - Rank the carbanions shown in the margin from most...Ch. 2.6 - Which is the stronger acid?Ch. 2.6 - Prob. 23PCh. 2.6 - What reaction in Problem 23 has the smallest...Ch. 2.6 - Rank the halide ions (F, Cl, Br, and l) from...Ch. 2.6 - a. Which is more electronegative, oxygen or...Ch. 2.6 - Which is a stronger acid? a. HCl or HBr b....Ch. 2.6 - a. Which of the halide ions (F, Cl, Br, and l) is...Ch. 2.6 - Which is a stronger base? (The potential maps in...Ch. 2.7 - What is a stronger acid? a. CH3OCH2CH2OH or...Ch. 2.7 - Rank the following compounds from strongest add to...Ch. 2.7 - What is a stronger base?Ch. 2.8 - For each of the following compounds, indicate the...Ch. 2.8 - Prob. 35PCh. 2.8 - Which is a stronger acid? Why?Ch. 2.8 - Fosamax (shown on the previous page) has six...Ch. 2.9 - Using the table of pKa values given in Appendix I,...Ch. 2.10 - For each of the following compounds (here shown in...Ch. 2.10 - As long as the pH is not less than _______, at...Ch. 2.10 - a. Indicate whether a protonated amine (RN+H3)...Ch. 2.10 - A naturally occurring amino acid such as alanine...Ch. 2.10 - a. At what pH is the concentration of a compound,...Ch. 2.10 - For each of the following compounds, indicate the...Ch. 2.10 - Given the data in Problem 47: a. What pH would you...Ch. 2.11 - Write the equation that shows how a buffer made by...Ch. 2.12 - Draw the products of the following react ions. Use...Ch. 2.12 - What product are formed when each of the following...Ch. 2 - Which is a stronger base? a. HS or HO b. CH3O or...Ch. 2 - According to the explanations by Lewis, if a...Ch. 2 - a. Rank the following alcohols from strongest to...Ch. 2 - a. Rank the following carboxylic acids from...Ch. 2 - Prob. 57PCh. 2 - For the following compound. a. draw its conjugate...Ch. 2 - Rank the following compounds from strongest to...Ch. 2 - Prob. 60PCh. 2 - Prob. 61PCh. 2 - a. Rank the following alcohols from strongest to...Ch. 2 - A single bond between two carbons with different...Ch. 2 - For each compound, indicate the atom that is most...Ch. 2 - a. Given the Ka values, estimate the pKa value of...Ch. 2 - Tenormin, a member of the group of drugs known as...Ch. 2 - From which of the following compounds can HO...Ch. 2 - a. For each of the following pairs of reactions,...Ch. 2 - Prob. 69PCh. 2 - Which is a stronger acid? a. b. c. d.Ch. 2 - Prob. 71PCh. 2 - Prob. 72PCh. 2 - Given that pH+ pOH = 14 and that the concentration...Ch. 2 - How could you separate a mixture of the following...Ch. 2 - Prob. 75PCh. 2 - a. If an add with a pKa of 5.3 is in an aqueous...Ch. 2 - Calculate the pH values of the following...Ch. 2 - Prob. 1PCh. 2 - Prob. 2PCh. 2 - Prob. 3PCh. 2 - Which of the reactions in Problem 3 favor...Ch. 2 - Prob. 5PCh. 2 - Prob. 6PCh. 2 - Prob. 7PCh. 2 - Which is the stronger acid? a. ClCH2CH2OH or...Ch. 2 - Prob. 9PCh. 2 - Prob. 10PCh. 2 - Which is a more stable base? Remembering that the...Ch. 2 - Which is the Stronger acid?Ch. 2 - Prob. 13PCh. 2 - a. Draw the structure of (CH3COOH (pKa = 4.7) at...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please list the String of Letters in the correct order.arrow_forward2. Propose an efficient synthesis for each of the following transformations. Pay careful attention to both the regio and stereochemical outcomes. ¡ H H racemicarrow_forwardZeroth Order Reaction In a certain experiment the decomposition of hydrogen iodide on finely divided gold is zeroth order with respect to HI. 2HI(g) Au H2(g) + 12(9) Rate = -d[HI]/dt k = 2.00x104 mol L-1 s-1 If the experiment has an initial HI concentration of 0.460 mol/L, what is the concentration of HI after 28.0 minutes? 1 pts Submit Answer Tries 0/5 How long will it take for all of the HI to decompose? 1 pts Submit Answer Tries 0/5 What is the rate of formation of H2 16.0 minutes after the reaction is initiated? 1 pts Submit Answer Tries 0/5arrow_forward
- Don't used Ai solutionarrow_forwardSaved v Question: I've done both of the graphs and generated an equation from excel, I just need help explaining A-B. Below is just the information I used to get the graphs obtain the graph please help. Prepare two graphs, the first with the percent transmission on the vertical axis and concentration on the horizontal axis and the second with absorption on the vertical axis and concentration on the horizontal axis. Solution # Unknown Concentration (mol/L) Transmittance Absorption 9.88x101 635 0.17 1.98x101 47% 0.33 2.95x101 31% 0.51 3.95x10 21% 0.68 4.94x10 14% 24% 0.85 0.62 A.) Give an equation that relates either the % transmission or the absorption to the concentration. Explain how you arrived at your equation. B.) What is the relationship between the percent transmission and the absorption? C.) Determine the concentration of the ironlll) salicylate in the unknown directly from the graph and from the best fit trend-line (least squares analysis) of the graph that yielded a straight…arrow_forwardDon't used Ai solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Biomolecules - Protein - Amino acids; Author: Tutorials Point (India) Ltd.;https://www.youtube.com/watch?v=ySNVPDHJ0ek;License: Standard YouTube License, CC-BY