
MASTERPHYS:KNIGHT'S PHYSICS ACCESS+WKB
4th Edition
ISBN: 9780135245033
Author: Knight
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 21, Problem 6CQ
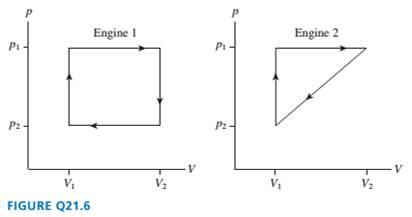
FIGURE Q21.6 shows the
engines. Which
are they the same? Explain.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
answer both questions
- 13-
3.
Shastri recalled reading that for an ideal transformer, "the ratio of the primary voltage to the
secondary voltage is equal to the ratio of the secondary current to the primary current."
Plan and design an experiment to investigate whether the statement above is true.
(8)
•
With the aid of a fully labelled circuit diagram, describe a procedure which can be used to
investigate whether the statement is true. The circuit diagram must include the following
components:
A variable AC voltage supply
•
AC voltmeters
•
AC ammeters
A transformer with adjustable turns ratio
Connecting wires
•
°
A load resistor
answer question 1-6
Chapter 21 Solutions
MASTERPHYS:KNIGHT'S PHYSICS ACCESS+WKB
Ch. 21 - Prob. 1CQCh. 21 - Rank in order, from largest to smallest, the...Ch. 21 - Prob. 3CQCh. 21 - FIGURE Q21.4 shows the pV diagram of a heat...Ch. 21 - Rank in order, from largest to smallest, the...Ch. 21 - FIGURE Q21.6 shows the thermodynamic cycles of two...Ch. 21 - A heat engine satisfies Wout= Qnet. Why is there...Ch. 21 - Prob. 8CQCh. 21 - Prob. 9CQCh. 21 - Prob. 10CQ
Ch. 21 - Prob. 11CQCh. 21 - Prob. 1EAPCh. 21 - Prob. 2EAPCh. 21 - Prob. 3EAPCh. 21 - Prob. 4EAPCh. 21 - Prob. 5EAPCh. 21 - Prob. 6EAPCh. 21 - The power output of a car engine running at 2400...Ch. 21 - Prob. 8EAPCh. 21 - Prob. 9EAPCh. 21 - Prob. 10EAPCh. 21 - Prob. 11EAPCh. 21 - Prob. 12EAPCh. 21 - Prob. 13EAPCh. 21 - Prob. 14EAPCh. 21 - Prob. 15EAPCh. 21 - Prob. 16EAPCh. 21 - A heat engine uses a diatomic gas in a Brayton...Ch. 21 - At what pressure ratio does a Brayton cycle using...Ch. 21 - Prob. 19EAPCh. 21 - Prob. 20EAPCh. 21 - Prob. 21EAPCh. 21 - Prob. 22EAPCh. 21 - Prob. 23EAPCh. 21 - Prob. 24EAPCh. 21 - Prob. 25EAPCh. 21 - Prob. 26EAPCh. 21 - Prob. 27EAPCh. 21 - A Carnot engine whose hot-reservoir temperature is...Ch. 21 - Prob. 29EAPCh. 21 - A heat engine operating between energy reservoirs...Ch. 21 - Prob. 31EAPCh. 21 - A Carnot refrigerator operating between —20°C and...Ch. 21 - The coefficient of performance of a refrigerator...Ch. 21 - A Carnot heat engine with thermal efficiency 1/3...Ch. 21 - Prob. 35EAPCh. 21 - Prob. 36EAPCh. 21 - A heat engine with 50% of the Carnot efficiency...Ch. 21 - Prove that the work done in an adiabatic process i...Ch. 21 - Prob. 39EAPCh. 21 - Prob. 40EAPCh. 21 - An ideal refrigerator utilizes a Carnot cycle...Ch. 21 - Prob. 42EAPCh. 21 - There has long been an interest in using the vast...Ch. 21 - A Carnot heat engine operates between reservoirs...Ch. 21 - A Carnot engine operates between temperatures of...Ch. 21 - Prob. 46EAPCh. 21 - A Carnot heat engine and an ordinary refrigerator...Ch. 21 - 48. A heat engine running backward is called a...Ch. 21 - 49. A car's internal combustion engine can be...Ch. 21 - Prob. 50EAPCh. 21 - Prob. 51EAPCh. 21 - Prob. 52EAPCh. 21 - Prob. 53EAPCh. 21 - Prob. 54EAPCh. 21 - Prob. 55EAPCh. 21 - Prob. 56EAPCh. 21 - Prob. 57EAPCh. 21 - A heat engine using a monatomic gas follows the...Ch. 21 - Prob. 59EAPCh. 21 - Prob. 60EAPCh. 21 - Prob. 61EAPCh. 21 - Prob. 62EAPCh. 21 - Prob. 63EAPCh. 21 - Prob. 64EAPCh. 21 - Prob. 65EAPCh. 21 - Prob. 66EAPCh. 21 - Prob. 67EAPCh. 21 - Prob. 68EAPCh. 21 - Prob. 69EAPCh. 21 - Prob. 70EAPCh. 21 - A refrigerator using helium gas operates on the...Ch. 21 - Prob. 72EAPCh. 21 - The gasoline engine in your car can be modeled as...Ch. 21 - Prob. 74EAP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Fractions 1. Covert 5/7 to a decimal 2. 5/7 x 3/8 3. 2/5 divided 4/9 4. covert 37/ 19 to a decimalarrow_forwardthis is an exam past paper question that i need help with becuase i am reviewing not a graded assignmentarrow_forwardsunny (1) -13- end. One box contains nothing inside; one has a piece of resistance wire between the terminals You are provided with three sealed identical matchboxes labelled A, B and C, with terminals at each and the other, a semi-conductor diode. Plan and design an experiment to identify the contents of each box. You are provided with the following elements for your apparatus: Ammeter Low voltage power supply Connecting wires Labelled circuit diagram Draw a well-labelled circuit diagram to show how you would connect the apparatus listed above to each matchbox. (3 maarrow_forward
- RAD127 Radiographic Equipment and Computers SI Units in Radiography Ch. 1 & 2 Instructions: Provide the units for each of the following in full and short forms 1. Mass - kg, 9 or (1b)) ・ 2. Energy, Work - W = FD,J 3. Air kerma -(Gya) 4. Absorbed Dose- 5. Effective Dose J/kg (94+) jlkg J/kg, Sv 6. Radioactivity - 5-1, Bq 7. Weight 8. Time 9. Force 10. Power B9 wt, wt-mg, N -(s) F= ma, N, OR 1b. (JIS), P= work It = Fdlt, Jarrow_forwardanswer 1-8arrow_forward1 . Solve the equation 2/7=y/3 for y. 2. Solve the equation x/9=2/6 for x. 3. Solve the equation z + 4 = 10 This is algebra and the equation is fraction.arrow_forward
- two satellites are in circular orbits around the Earth. Satellite A is at an altitude equal to the Earth's radius, while satellite B is at an altitude equal to twice the Earth's radius. What is the ratio of their periods, Tb/Taarrow_forwardFresnel lens: You would like to design a 25 mm diameter blazed Fresnel zone plate with a first-order power of +1.5 diopters. What is the lithography requirement (resolution required) for making this lens that is designed for 550 nm? Express your answer in units of μm to one decimal point. Fresnel lens: What would the power of the first diffracted order of this lens be at wavelength of 400 nm? Express your answer in diopters to one decimal point. Eye: A person with myopic eyes has a far point of 15 cm. What power contact lenses does she need to correct her version to a standard far point at infinity? Give your answer in diopter to one decimal point.arrow_forwardParaxial design of a field flattener. Imagine your optical system has Petzal curvature of the field with radius p. In Module 1 of Course 1, a homework problem asked you to derive the paraxial focus shift along the axis when a slab of glass was inserted in a converging cone of rays. Find or re-derive that result, then use it to calculate the paraxial radius of curvature of a field flattener of refractive index n that will correct the observed Petzval. Assume that the side of the flattener facing the image plane is plano. What is the required radius of the plano-convex field flattener? (p written as rho )arrow_forward
- 3.37(a) Five free electrons exist in a three-dimensional infinite potential well with all three widths equal to \( a = 12 \, \text{Å} \). Determine the Fermi energy level at \( T = 0 \, \text{K} \). (b) Repeat part (a) for 13 electrons. Book: Semiconductor Physics and Devices 4th ed, NeamanChapter-3Please expert answer only. don't give gpt-generated answers, & please clear the concept of quantum states for determining nx, ny, nz to determine E, as I don't have much idea about that topic.arrow_forward3.37(a) Five free electrons exist in a three-dimensional infinite potential well with all three widths equal to \( a = 12 \, \text{Å} \). Determine the Fermi energy level at \( T = 0 \, \text{K} \). (b) Repeat part (a) for 13 electrons. Book: Semiconductor Physics and Devices 4th ed, NeamanChapter-3Please expert answer only. don't give gpt-generated answers, & please clear the concept of quantum states for determining nx, ny, nz to determine E, as I don't have much idea about that topic.arrow_forwardNo chatgpt pls will upvotearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning
The Second Law of Thermodynamics: Heat Flow, Entropy, and Microstates; Author: Professor Dave Explains;https://www.youtube.com/watch?v=MrwW4w2nAMc;License: Standard YouTube License, CC-BY