
(a)
The number of moles in the sample.
(a)
Answer to Problem 65AP
The number of moles in the sample is
Explanation of Solution
Write the expression from
Here,
Rearrange the above equation for
Conclusion:
Substitute
Therefore, the number of moles in the sample is
(b)
The temperature of the gas at point B.
(b)
Answer to Problem 65AP
The temperature of the gas at point B is
Explanation of Solution
Write the expression for temperature of the sample gas at point B.
Here,
Conclusion:
Substitute
Therefore, the temperature of the gas at point B is
(c)
The temperature of the gas at point C.
(c)
Answer to Problem 65AP
The temperature of the gas at point C is
Explanation of Solution
The temperature of the gas at point C is equal to the temperature at point B.
Conclusion:
Substitute
Therefore, the temperature of the gas at point C is
(d)
The volume at the point C.
(d)
Answer to Problem 65AP
The volume at the point C is
Explanation of Solution
The figure 1 shows the PV diagram.
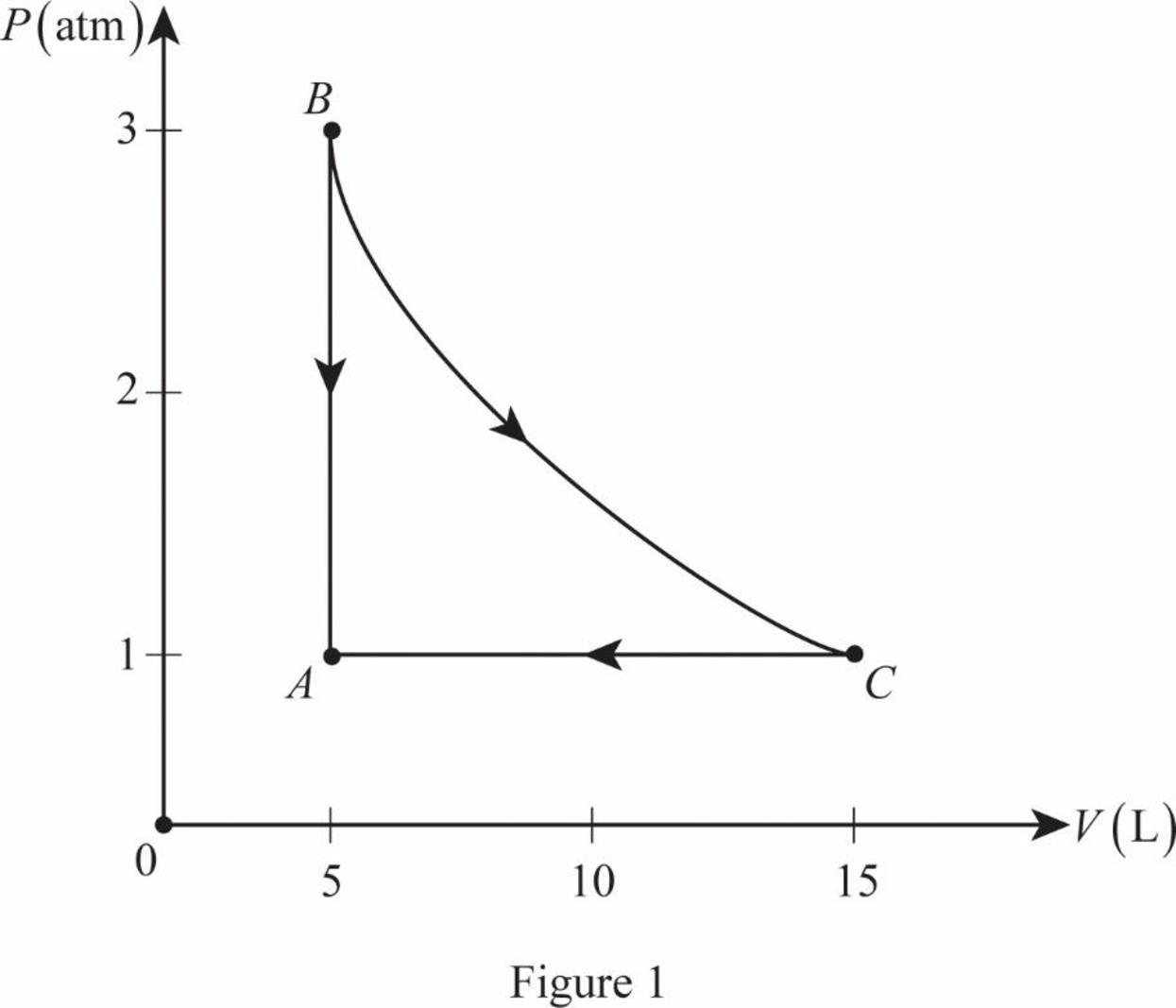
Write the expression for volume of the sample gas at point C.
Here,
Conclusion:
Substitute
Therefore, the volume at the point C is
(e)
How to carry out the process
(e)
Answer to Problem 65AP
The process
Explanation of Solution
Conclusion:
(f)
Find
(f)
Answer to Problem 65AP
The
Explanation of Solution
For
Find the internal energy of the gas.
Here,
Find the heat energy for the given process.
For
Find the work done on the gas.
Find the heat energy for the given process.
For
Find the internal energy of the gas.
Find the work done on the gas.
Find the heat energy for the given process.
Conclusion:
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Therefore, the
(g)
Find
(g)
Answer to Problem 65AP
The whole cycle process
Explanation of Solution
Find the heat energy for the whole cycle process.
Find the work done on the gas for the whole cycle process.
Find the internal energy of the gas for the whole cycle process.
Conclusion:
Substitute
Substitute
Substitute
Therefore, the whole cycle process
Want to see more full solutions like this?
Chapter 21 Solutions
Physics For Scientists And Engineers With Modern Physics, 9th Edition, The Ohio State University
- A pendulum bob A (0.5 kg) is given an initialspeed of vA = 4 m/s when the chord ishorizontal. It then hits a stationary block B (1kg) which then slides to a maximum distanced before it stops. Determine the value of d.The coefficient of static friction between theblock and the plane is μk = 0.2. The coefficientof restitution between A and B is e = 0.8.Ans: d=1.0034 marrow_forwardFigure 29-43 Problem 12. ••13 In Fig. 29-44, point P₁ is at distance R = 13.1 cm on the perpendicular bisector of a straight wire of length L = 18.0 cm carrying current i = 58.2 mA. (Note that the wire is not long.) What is the magnitude of the magnetic field at P₁ due to i? P2° R R Larrow_forwardCheckpoint 1 The figure shows the current i in a single-loop circuit with a battery B and a resistance R (and wires of neg- ligible resistance). (a) Should the emf arrow at B be drawn pointing leftward or rightward? At points a, B C R b, and c, rank (b) the magnitude of the current, (c) the electric potential, and (d) the electric potential energy of the charge carriers, greatest first.arrow_forward
- 3. If the force of gravity stopped acting on the planets in our solar system, what would happen? a) They would spiral slowly towards the sun. b) They would continue in straight lines tangent to their orbits. c) They would continue to orbit the sun. d) They would fly straight away from the sun. e) They would spiral slowly away from the sun. 4. 1 The free-body diagram of a wagon being pulled along a horizontal surface is best represented by A F N B C 0 Ꭰ FN E a) A b) B c) C app app The app 10 app d) e) ס ח D E 10 apparrow_forwardPls help ASAParrow_forwardPls help asaparrow_forward
 Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning





