
Organic Chemistry Study Guide and Solutions Manual, Books a la Carte Edition (8th Edition)
8th Edition
ISBN: 9780134649771
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 21, Problem 61P
Interpretation Introduction
Interpretation:
The difference in the
Concept introduction:
The value of
If the acidity or easiness of the protonation is high for a compound, then the value of the
The structure of alanine is,
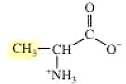
The structure of serine is,
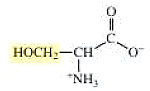
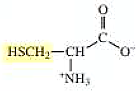
The structure of cysteine is,
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Pls help asap
16. Provide the correct IUPAC name for each of the following organic chemical compounds
a.
Pls help asap
Chapter 21 Solutions
Organic Chemistry Study Guide and Solutions Manual, Books a la Carte Edition (8th Edition)
Ch. 21.1 - a. Explain why, when the imidazole ring of...Ch. 21.2 - a. Which isomer(R)-alanine or (S)-alanineis...Ch. 21.2 - Prob. 4PCh. 21.3 - Prob. 5PCh. 21.3 - Prob. 6PCh. 21.3 - Draw the predominant form for glutamate in a...Ch. 21.3 - a. Why is the pKa of the glutamate side chain...Ch. 21.4 - Calculate the pI of each of the following amino...Ch. 21.4 - a. Which amino acid has the lowest pI value? b....Ch. 21.4 - Prob. 12P
Ch. 21.4 - Prob. 13PCh. 21.4 - Explain why the pI of lysine is the average of the...Ch. 21.5 - What aldehyde is formed when valine is treated...Ch. 21.5 - Prob. 16PCh. 21.5 - Prob. 17PCh. 21.5 - Prob. 18PCh. 21.5 - Prob. 19PCh. 21.6 - Why is excess ammonia used in the preceding...Ch. 21.6 - Prob. 21PCh. 21.6 - What amino acid is formed using the...Ch. 21.6 - Prob. 23PCh. 21.6 - What amino acid is formed when the aldehyde used...Ch. 21.7 - Esterase is an enzyme that catalyzes the...Ch. 21.8 - Draw the tetrapeptide Ala-Thr-Asp-Asn and indicate...Ch. 21.8 - Draw the resonance contributors of the peptide...Ch. 21.8 - Which bonds in the backbone of a peptide can...Ch. 21.9 - An opioid pentapeptide has the following...Ch. 21.9 - What is the configuration about each of the...Ch. 21.9 - Glutathione is a tripeptide whose function is to...Ch. 21.10 - What dipeptides would be formed by heating a...Ch. 21.10 - Suppose you are trying to synthesize the dipeptide...Ch. 21.10 - Show the steps in the synthesis of the...Ch. 21.10 - a. Calculate the overall yield of bradykinin when...Ch. 21.11 - Show the steps in the synthesis of the...Ch. 21.13 - Prob. 37PCh. 21.13 - In determining the primary structure of insulin,...Ch. 21.13 - A decapeptide undergoes partial hydrolysis to give...Ch. 21.13 - Explain why cyanogen bromide does not cleave on...Ch. 21.13 - Indicate the peptides produced from cleavage by...Ch. 21.14 - Prob. 43PCh. 21.14 - Three peptides were obtained from a trypsin...Ch. 21.14 - Prob. 45PCh. 21.15 - How would a protein that resides in the nonpolar...Ch. 21.16 - a. Which would have the greatest percentage of...Ch. 21.17 - When apples that have been cut are exposed to...Ch. 21 - Glycine has pK2 values of 2.34 and 9.60. At what...Ch. 21 - Prob. 50PCh. 21 - A titration curve is a plot of the pH of a...Ch. 21 - Prob. 52PCh. 21 - Aspartame (its structure is on page 1007) has a pl...Ch. 21 - Draw the form of aspartate that predominates at...Ch. 21 - Show how phenylalanine can be prepared by...Ch. 21 - A professor was preparing a manuscript for...Ch. 21 - What aldehydes are formed when the following amino...Ch. 21 - Prob. 58PCh. 21 - Determine the amino acid sequence of a polypeptide...Ch. 21 - Prob. 60PCh. 21 - Prob. 61PCh. 21 - Which is the more effective buffer at...Ch. 21 - Identify the location and type of charge on the...Ch. 21 - Draw the product obtained when a lysine side chain...Ch. 21 - After the polypeptide shown below was treated with...Ch. 21 - Treatment of a polypeptide with 2-mercaptoethanol...Ch. 21 - Show how aspartame can be synthesized using DCCD.Ch. 21 - -Amino acids can be prepared by treating an...Ch. 21 - Reaction of a polypeptide with carboxypeptidase A...Ch. 21 - a. How many different octapeptides can be made...Ch. 21 - Glycine has pKa values of 2.3 and 9.6. Do you...Ch. 21 - A mixture of 15 amino acids gave the fingerprint...Ch. 21 - Write the mechanism for the reaction of an amino...Ch. 21 - Prob. 74PCh. 21 - Show how valine can be prepared by a. a...Ch. 21 - The primary structure of -endorphin, a peptide...Ch. 21 - A chemist wanted to test his hypothesis that the...Ch. 21 - Propose a mechanism for the rearrangement of the...Ch. 21 - A normal polypeptide and a mutant of the...Ch. 21 - Determine the amino acid sequence of a polypeptide...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Pls help asaparrow_forwardPredict the major products of this reaction: ་ ་ + H NaOH ? Δ excess Note that the second reactant is used in excess, that is, there is much more of the second reactant than the first. If there won't be any products, just check the box under the drawing area instead.arrow_forwardP A student claims the right-hand side of the reaction in the drawing area below shows the product of a Claisen condensation. • If the student is correct, complete the reaction by adding the necessary organic reactants to the left-hand side, and by adding any necessary reagents and reaction conditions above and below the arrow. • If the student is incorrect, because it's not possible to obtain this product from a Claisen condensation, check the box under the drawing area instead. those that will minimize any byproducts or competing • Note for advanced students: If you have a choice, use the most efficient reactants and reagents reactions. - ☐ ☐ : ☐ + I Х Click and drag to start drawing a structure.arrow_forward
- identify the relationship between the structures and H- OH HO H H- OH and HO H H -ОН HO H Br and Brarrow_forwardThe right-hand side of this reaction shows the product of an aldol condensation. What are the reactants missing from the left-hand side? Draw them below. ? NaOH Δ If there aren't any reactants that would lead to these products under the reaction conditions given, just check the box under the drawing area. Note for advanced students: don't worry if the reactants you propose might also make some other products under these reaction conditions. Just make sure the product above is one of the major products.arrow_forwardPlease help! I need to identify four labeled unknown bottles based off of their colors doing titration using phenlphtalein. I've included my answers, but I wanted to make sure they were correct and if not, what will be correct thank you in advance.arrow_forward
- An organic chemistry Teaching Assistant (TA) suggested in your last discussion section that there is only one major organic product of the following reaction and that this reaction builds a ring. If the TA is right, draw the product in the drawing area below. If the TA is wrong, just check the box below the drawing area. NaOH ?arrow_forwardA student suggests that the molecule on the right can be made from a single molecule that doesn't have a ring. If the student is correct, draw the starting material below, otherwise, check the box under the drawing area. Click and drag to start drawing a structure. : ☐ + NaOH टेarrow_forwardRate = k [I]1.7303[S2O82-]0.8502, Based on your rate, write down a mechanism consistent with your results and indicate which step is the rate determining step.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning