
Student Workbook for Physics for Scientists and Engineers: A Strategic Approach, Vol 1. (Chs 1-21)
4th Edition
ISBN: 9780134110646
Author: Randall D. Knight (Professor Emeritus)
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 21, Problem 5CQ
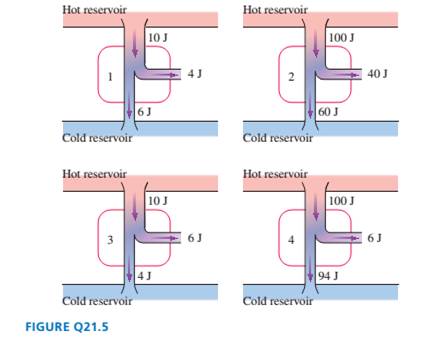
Rank in order, from largest to smallest, the thermal efficiencies
?1to ?2 of the four
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
No chatgpt pls will upvote
No chatgpt pls will upvote
No chatgpt pls
Chapter 21 Solutions
Student Workbook for Physics for Scientists and Engineers: A Strategic Approach, Vol 1. (Chs 1-21)
Ch. 21 - Prob. 1CQCh. 21 - Rank in order, from largest to smallest, the...Ch. 21 - Prob. 3CQCh. 21 - FIGURE Q21.4 shows the pV diagram of a heat...Ch. 21 - Rank in order, from largest to smallest, the...Ch. 21 - FIGURE Q21.6 shows the thermodynamic cycles of two...Ch. 21 - A heat engine satisfies Wout= Qnet. Why is there...Ch. 21 - Prob. 8CQCh. 21 - Prob. 9CQCh. 21 - Prob. 10CQ
Ch. 21 - Prob. 11CQCh. 21 - Prob. 1EAPCh. 21 - Prob. 2EAPCh. 21 - Prob. 3EAPCh. 21 - Prob. 4EAPCh. 21 - Prob. 5EAPCh. 21 - Prob. 6EAPCh. 21 - The power output of a car engine running at 2400...Ch. 21 - Prob. 8EAPCh. 21 - Prob. 9EAPCh. 21 - Prob. 10EAPCh. 21 - Prob. 11EAPCh. 21 - Prob. 12EAPCh. 21 - Prob. 13EAPCh. 21 - Prob. 14EAPCh. 21 - Prob. 15EAPCh. 21 - Prob. 16EAPCh. 21 - A heat engine uses a diatomic gas in a Brayton...Ch. 21 - At what pressure ratio does a Brayton cycle using...Ch. 21 - Prob. 19EAPCh. 21 - Prob. 20EAPCh. 21 - Prob. 21EAPCh. 21 - Prob. 22EAPCh. 21 - Prob. 23EAPCh. 21 - Prob. 24EAPCh. 21 - Prob. 25EAPCh. 21 - Prob. 26EAPCh. 21 - Prob. 27EAPCh. 21 - A Carnot engine whose hot-reservoir temperature is...Ch. 21 - Prob. 29EAPCh. 21 - A heat engine operating between energy reservoirs...Ch. 21 - Prob. 31EAPCh. 21 - A Carnot refrigerator operating between —20°C and...Ch. 21 - The coefficient of performance of a refrigerator...Ch. 21 - A Carnot heat engine with thermal efficiency 1/3...Ch. 21 - Prob. 35EAPCh. 21 - Prob. 36EAPCh. 21 - A heat engine with 50% of the Carnot efficiency...Ch. 21 - Prove that the work done in an adiabatic process i...Ch. 21 - Prob. 39EAPCh. 21 - Prob. 40EAPCh. 21 - An ideal refrigerator utilizes a Carnot cycle...Ch. 21 - Prob. 42EAPCh. 21 - There has long been an interest in using the vast...Ch. 21 - A Carnot heat engine operates between reservoirs...Ch. 21 - A Carnot engine operates between temperatures of...Ch. 21 - Prob. 46EAPCh. 21 - A Carnot heat engine and an ordinary refrigerator...Ch. 21 - 48. A heat engine running backward is called a...Ch. 21 - 49. A car's internal combustion engine can be...Ch. 21 - Prob. 50EAPCh. 21 - Prob. 51EAPCh. 21 - Prob. 52EAPCh. 21 - Prob. 53EAPCh. 21 - Prob. 54EAPCh. 21 - Prob. 55EAPCh. 21 - Prob. 56EAPCh. 21 - Prob. 57EAPCh. 21 - A heat engine using a monatomic gas follows the...Ch. 21 - Prob. 59EAPCh. 21 - Prob. 60EAPCh. 21 - Prob. 61EAPCh. 21 - Prob. 62EAPCh. 21 - Prob. 63EAPCh. 21 - Prob. 64EAPCh. 21 - Prob. 65EAPCh. 21 - Prob. 66EAPCh. 21 - Prob. 67EAPCh. 21 - Prob. 68EAPCh. 21 - Prob. 69EAPCh. 21 - Prob. 70EAPCh. 21 - A refrigerator using helium gas operates on the...Ch. 21 - Prob. 72EAPCh. 21 - The gasoline engine in your car can be modeled as...Ch. 21 - Prob. 74EAP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- 4.4 A man is dragging a trunk up the loading ramp of a mover's truck. The ramp has a slope angle of 20.0°, and the man pulls upward with a force F whose direction makes an angle of 30.0° 75.0° with the ramp (Fig. E4.4). (a) How large a force F is necessary for the component Fx parallel to the ramp to be 90.0 N? (b) How large will the component Fy perpendicular to the ramp be then? Figure E4.4 30.0 20.0°arrow_forward1. * A projectile is shot from a launcher at an angle e, with an initial velocity magnitude v., from a point even with a tabletop. The projectile lands on the tabletop a horizontal distance R (the "range") away from where it left the launcher. Set this up as a formal problem, and solve for vo (i.e., determine an expression for Vo in terms of only R, 0., and g). Your final equation will be called Equation 1.arrow_forward2. A projectile is shot from a launcher at an angle 0,, with an initial velocity magnitude vo, from a point even with a tabletop. The projectile hits an apple atop a child's noggin (see Figure 1). The apple is a height y above the tabletop, and a horizontal distance x from the launcher. Set this up as a formal problem, and solve for x. That is, determine an expression for x in terms of only v₁, o,y and g. Actually, this is quite a long expression. So, if you want, you can determine an expression for x in terms of v., 0., and time t, and determine another expression for timet (in terms of v., 0., y and g) that you will solve and then substitute the value of t into the expression for x. Your final equation(s) will be called Equation 3 (and Equation 4).arrow_forward
- 4.56 ... CALC An object of mass m is at rest in equilibrium at the origin. At t = 0 a new force F(t) is applied that has components Fx(t) = k₁ + k₂y Fy(t) = k3t where k₁, k2, and k3 are constants. Calculate the position (1) and veloc- ity (t) vectors as functions of time.arrow_forward4.14 ⚫ A 2.75 kg cat moves in a straight line (the x-axis). Figure E4.14 shows a graph of the x- component of this cat's velocity as a function of time. (a) Find the maximum net force on this cat. When does this force occur? (b) When is the net force on the cat equal to zero? (c) What is the net force at time 8.5 s? Figure E4.14 V₁ (m/s) 12.0 10.0 8.0 6.0 4.0 2.0 0 t(s) 2.0 4.0 6.0 8.0 10.0arrow_forward4.36 ... CP An advertisement claims that a particular automobile can "stop on a dime." What net force would be necessary to stop a 850 kg automobile traveling initially at 45.0 km/h in a distance equal to the di- ameter of a dime, 1.8 cm?arrow_forward
- 4.46 The two blocks in Fig. P4.46 are connected by a heavy uniform rope with a mass of 4.00 kg. An up- ward force of 200 N is applied as shown. (a) Draw three free-body diagrams: one for the 6.00 kg block, one for B the 4.00 kg rope, and another one for the 5.00 kg block. For each force, indicate what object exerts that force. (b) What is the acceleration of the system? (c) What is the tension at the top of the heavy rope? (d) What is the tension at the midpoint of the rope? Figure P4.46 F= 200 N 4.00 kg 6.00 kg 5.00 kgarrow_forward4.35 ⚫ Two adults and a child want to push a wheeled cart in the direc- tion marked x in Fig. P4.35 (next page). The two adults push with hori- zontal forces F and F as shown. (a) Find the magnitude and direction of the smallest force that the child should exert. Ignore the effects of friction. (b) If the child exerts the minimum force found in part (a), the cart ac- celerates at 2.0 m/s² in the +x-direction. What is the weight of the cart? Figure P4.35 F₁ = 100 N 60° 30° F2 = 140 Narrow_forward4.21 ⚫ BIO World-class sprinters can accelerate out of the starting blocks with an acceleration that is nearly horizontal and has magnitude 15 m/s². How much horizontal force must a 55 kg sprinter exert on the starting blocks to produce this acceleration? Which object exerts the force that propels the sprinter: the blocks or the sprinter herself?arrow_forward
- No chatgpt pls will upvotearrow_forwardPlease don't use Chatgpt will upvote and give handwritten solutionarrow_forwardThe kinetic energy of a pendulum is greatest Question 20Select one: a. at the top of its swing. b. when its potential energy is greatest. c. at the bottom of its swing. d. when its total energy is greatest.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
The Second Law of Thermodynamics: Heat Flow, Entropy, and Microstates; Author: Professor Dave Explains;https://www.youtube.com/watch?v=MrwW4w2nAMc;License: Standard YouTube License, CC-BY