
ORGANIC CHEM W/BIOLOGICAL TOP. ACCESS
6th Edition
ISBN: 9781264382545
Author: SMITH
Publisher: MCG CUSTOM
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21, Problem 38P
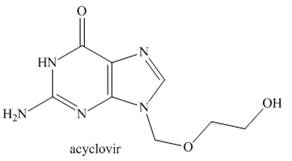
Acyclovir is an effective antiviral agent used to treat the herpes simplex virus.(a) Draw the enol form of acyclovir, and explain why it is
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Briefly describe the compounds called carboranes.
Please don't use Ai solution
None
Chapter 21 Solutions
ORGANIC CHEM W/BIOLOGICAL TOP. ACCESS
Ch. 21.2 - Problem 23.1 Draw the enol or keto tautomer(s) of...Ch. 21.2 - Problem 23.3 When phenylacetaldehyde is dissolved...Ch. 21.3 - Prob. 5PCh. 21.3 - Problem 23.5 Which bonds in the following...Ch. 21.3 - Prob. 7PCh. 21.3 - Prob. 8PCh. 21.3 - Prob. 9PCh. 21.4 - Prob. 10PCh. 21.5 - Prob. 11PCh. 21.7 - Problem 23.11 Draw the products of each...
Ch. 21.7 - Problem 23.12 Draw the products of each reaction....Ch. 21.7 - Prob. 14PCh. 21.7 - Prob. 15PCh. 21.8 - Prob. 16PCh. 21.8 - Prob. 17PCh. 21.8 - Prob. 18PCh. 21.8 - Problem 23.18 How can pentan-2-one be converted...Ch. 21.8 - Problem 23.19 Identify A, B, and C, intermediates...Ch. 21.9 - Problem 23.20 Which of the following compounds...Ch. 21.9 - Problem 23.21 Draw the products of each...Ch. 21.9 - Prob. 23PCh. 21.9 - Prob. 24PCh. 21.9 - Prob. 25PCh. 21.10 - Prob. 26PCh. 21.10 - Prob. 27PCh. 21.10 - Prob. 28PCh. 21.10 - Prob. 29PCh. 21 - 23.29 Draw enol tautomer(s) for each compound....Ch. 21 - 22.30 The cis ketone A is isomerized to a trans...Ch. 21 - 23.31 Draw enol tautomer(s) for each compound.
...Ch. 21 - Prob. 33PCh. 21 - Prob. 34PCh. 21 - 23.35 Rank the labeled protons in each compound in...Ch. 21 - Prob. 36PCh. 21 - Prob. 37PCh. 21 - 23.38 Acyclovir is an effective antiviral agent...Ch. 21 - 23.39 Explain why forms two different alkylation...Ch. 21 - Prob. 40PCh. 21 - 23.42 Draw a stepwise mechanism for the following...Ch. 21 - Prob. 42PCh. 21 - Prob. 43PCh. 21 - 23.45 Devise a synthesis of valproic acid , a...Ch. 21 - Prob. 57PCh. 21 - 23.57 Draw a stepwise mechanism showing how two...Ch. 21 - 23.58 Draw a stepwise mechanism for the following...Ch. 21 - Prob. 65PCh. 21 - 23.66 Synthesize (Z)-hept-5-en-2-one from ethyl...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nonearrow_forwardJON Determine the bund energy for UCI (in kJ/mol Hcl) using me balanced chemical equation and bund energies listed? का (My (9) +36/2(g)-(((3(g) + 3(g) A Hryn = -330. KJ bond energy и-н 432 bond bond C-1413 C=C 839 N-H 391 C=O 1010 S-H 363 б-н 467 02 498 N-N 160 N=N 243 418 C-C 341 C-0 358 C=C C-C 339 N-Br 243 Br-Br C-Br 274 193 614 (-1 214||(=olin (02) 799 C=N 615 AALarrow_forwardDetermine the bond energy for HCI ( in kJ/mol HCI) using he balanced cremiculequecticnand bund energles listed? also c double bond to N is 615, read numbets carefully please!!!! Determine the bund energy for UCI (in kJ/mol cl) using me balanced chemical equation and bund energies listed? 51 (My (9) +312(g)-73(g) + 3(g) =-330. KJ спод bond energy Hryn H-H bond band 432 C-1 413 C=C 839 NH 391 C=O 1010 S-1 343 6-H 02 498 N-N 160 467 N=N C-C 341 CL- 243 418 339 N-Br 243 C-O 358 Br-Br C=C C-Br 274 193 614 (-1 216 (=olin (02) 799 C=N 618arrow_forward
- Differentiate between single links and multicenter links.arrow_forwardI need help on my practice final, if you could explain how to solve this that would be extremely helpful for my final thursday. Please dumb it down chemistry is not my strong suit. If you could offer strategies as well to make my life easier that would be beneficialarrow_forwardNonearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY