
(a)
Interpretation:
Synthesis of the given compound is to be shown.
Concept introduction:
The acetic acid is treated with various reagents to form products that contain different
Answer to Problem 21.85P
The synthesis of the given compound is as follows:
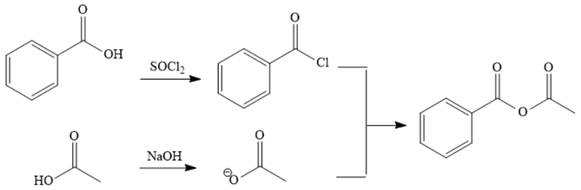
Explanation of Solution
The reaction requires converting a carboxylic acid to an acid anhydride, which represents going up the stability ladder. This is difficult to do directly but can be carried out by first producing an acid chloride. The acid chloride and the salt of acetic acid will make the desired anhydride.
The acetic acid is treated with various reagents to form products that contain different functional groups.
(b)
Interpretation:
The synthesis of the given compound is to be shown.
Concept introduction:
The acetic acid is treated with various reagents to form products that contain different functional groups. The new functional groups formed are anhydride, amine, ether, aldehyde, ketone, etc. When carboxylic acid converted to an acid anhydride, the stability ladder going up. When an anhydride reacted with ethanol to formed the ester, the stability ladder going down.
Answer to Problem 21.85P
The synthesis of the given compound is as follows:
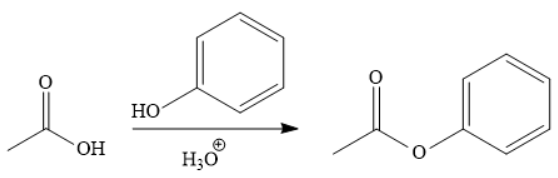
Explanation of Solution
The addition of phenol forms the ester under acidic condition, a Fischer esterification. This is a reversible reaction and can be carried out directly because the reactant and product species are on the same rung of the stability ladder.
The acetic acid is treated with various reagents to form products that contain different functional groups.
(c)
Interpretation:
The synthesis of the given compound is to be shown.
Concept introduction:
The acetic acid is treated with various reagents to form products that contain different functional groups. The new functional groups formed are anhydride, amine, ether, aldehyde, ketone, etc. When carboxylic acid converted to an acid anhydride, the stability ladder going up. When an anhydride reacted with ethanol to formed the ester, the stability ladder going down.
Answer to Problem 21.85P
The synthesis of the given compound is as follows,
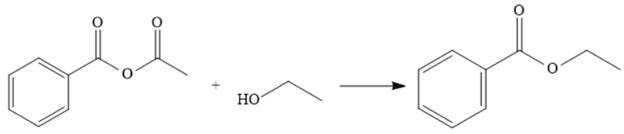
Explanation of Solution
The reaction of the anhydride from (a) with ethanol makes the ester. This represents going down the stability ladder and is done relatively easily.
The acetic acid is treated with various reagents to form products that contain different functional groups.
(d)
Interpretation:
The synthesis of the given compound is to be shown.
Concept introduction:
The acetic acid is treated with various reagents to form products that contain different functional groups. The new functional groups formed are anhydride, amine, ether, aldehyde, ketone, etc. When carboxylic acid converted to an acid anhydride, the stability ladder going up. When an anhydride reacted with ethanol to formed the ester, the stability ladder going down.
Answer to Problem 21.85P
The synthesis of the given compound is as follows:
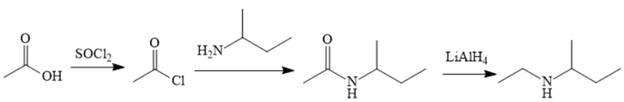
Explanation of Solution
The amine can be produced by reducing the corresponding amide. It is difficult to produce the amide directly from acetic acid because the amine that would be required for the nucleophile would deprotonate the carboxylic acid. The amide can be made relatively easily, however, by first converting acetic acid to acetyl chloride and then treating acetyl chloride with the amine.
The acetic acid is treated with various reagents to form products that contain different functional groups.
(e)
Interpretation:
The synthesis of the given compound is to be shown.
Concept introduction:
The acetic acid is treated with various reagents to form products that contain different functional groups. The new functional groups formed are anhydride, amine, ether, aldehyde, ketone, etc. When carboxylic acid converted to an acid anhydride, the stability ladder going up. When an anhydride reacted with ethanol to formed the ester, the stability ladder going down.
Answer to Problem 21.85P
The synthesis of the given compound is as follows,
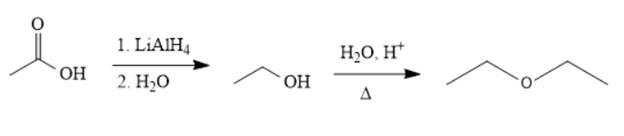
Explanation of Solution
Reducing the acid to an alcohol, followed by dehydration, leads to the ether. Alternatively, ethanol can be deprotonated by
The acetic acid is treated with various reagents to form products that contain different functional groups.
(f)
Interpretation:
The synthesis of the given compound is to be shown.
Concept introduction:
The acetic acid is treated with various reagents to form products that contain different functional groups. The new functional groups formed are anhydride, amine, ether, aldehyde, ketone, etc. When carboxylic acid converted to an acid anhydride, the stability ladder going up. When an anhydride reacted with ethanol to formed the ester, the stability ladder going down.
Answer to Problem 21.85P
The synthesis of the given compound is as follows,
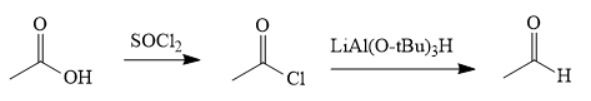
Explanation of Solution
First, acid is treated with
The acetic acid is treated with various reagents to form products which contain different functional groups.
(g)
Interpretation:
The synthesis of the given compound is to be shown.
Concept introduction:
The acetic acid is treated with various reagents to form products that contain different functional groups. The new functional groups formed are anhydride, amine, ether, aldehyde, ketone, etc. When carboxylic acid converted to an acid anhydride, the stability ladder going up. When an anhydride reacted with ethanol to formed the ester, the stability ladder going down.
Answer to Problem 21.85P
The synthesis of the given compound is as follows,
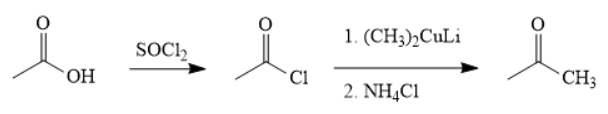
Explanation of Solution
First, acid is treated with
The acetic acid is treated with various reagents to form products which contain different functional groups.
Want to see more full solutions like this?
Chapter 21 Solutions
Organic Chemistry: Principles And Mechanisms (second Edition)
- CH3O OH OH O hemiacetal O acetal O neither O 0 O hemiacetal acetal neither OH hemiacetal O acetal O neither CH2 O-CH2-CH3 CH3-C-OH O hemiacetal O acetal CH3-CH2-CH2-0-c-O-CH2-CH2-CH3 O neither HO-CH2 ? 000 Ar Barrow_forwardWhat would be the best choices for the missing reagents 1 and 3 in this synthesis? 1. PPh3 2 2. n-BuLi 3 Draw the missing reagents in the drawing area below. You can draw them in any arrangement you like. • Do not draw the missing reagent 2. If you draw 1 correctly, we'll know what it is. • Note: if one of your reagents needs to contain a halogen, use bromine. Explanation Check Click and drag to start drawing a structure.arrow_forwardPredict the products of this organic reaction: NaBH3CN + NH2 ? H+ Click and drag to start drawing a structure. ×arrow_forward
- Predict the organic products that form in the reaction below: + OH +H H+ ➤ ☑ X - Y Note: You may assume you have an excess of either reactant if the reaction requires more than one of those molecules to form the products. In the drawing area below, draw the skeletal ("line") structures of the missing organic products X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Click and drag to start drawing a structure. Garrow_forwardPredict the organic products that form in the reaction below: OH H+ H+ + ☑ Y Note: You may assume you have an excess of either reactant if the reaction requires more than one of those molecules to form the products. In the drawing area below, draw the skeletal ("line") structures of the missing organic products X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Click and drag to start drawing a structure. ✓ marrow_forwardDetermine the structures of the missing organic molecules in the following reaction: + H₂O +H H+ Y Z ☑ ☑ Note: Molecules that share the same letter have the exact same structure. In the drawing area below, draw the skeletal ("line") structures of the missing organic molecules X, Y, and Z. You may draw the structures in any arrangement that you like, so long as they aren't touching. Molecule X shows up in multiple steps, but you only have to draw its structure once. Click and drag to start drawing a structure. AP +arrow_forward
- Please help, this is all the calculations i got!!! I will rate!!!Approx mass of KMnO in vial: 3.464 4 Moss of beaker 3×~0. z Nax200: = 29.9219 Massof weacerv after remosimgain N2C2O4. Need to fill in all the missing blanks. ง ง Approx mass of KMnO4 in vials 3.464 Mass of beaker + 3x ~0-304: 29.9219 2~0.20 Miss of beaker + 2x- 29.7239 Mass of beaker + 1x~0.2g Naz (204 29-5249 Mass of beaver after removing as qa Na₂ C₂O T1 T2 T3 Final Buiet reading Initial butet reading (int)) Hass of NaOr used for Titration -reading (mL) calculation Results: 8.5ml 17mL 27.4mL Oml Om Oml T1 T2 T3 Moles of No CO Moles of KMO used LOF KM. O used Molenty of KMNO Averagem Of KMOWLarrow_forwardDraw the skeletal ("line") structure of 2-hydroxy-4-methylpentanal. Click and drag to start drawing a structure. Xarrow_forwardDetermine whether the following molecule is a hemiacetal, acetal, or neither and select the appropriate box below. Also, highlight the hemiacetal or acetal carbon if there is one. hemiacetal acetal Oneither OHarrow_forward
- What is the missing reactant R in this organic reaction? ་ ་ ་ ་ ་ ་ ་ ་ ་ ་ +R H3O+ • Draw the structure of R in the drawing area below. N • Be sure to use wedge and dash bonds if it's necessary to draw one particular enantiomer. Click and drag to start drawing a structure.arrow_forwardWrite the systematic name of each organic molecule: H structure H OH OH H OH name ☐ OHarrow_forwardDetermine whether each of the following molecules is a hemiacetal, acetal, or neither and select the appropriate box in the table. CH3O OH OH OH hemiacetal acetal neither hemiacetal acetal neither Xarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





