
A sample of a monatomic ideal gas occupies 5.00 L at atmospheric pressure and 300 K (point A in Fig. P21.65). It is warmed at constant volume to 3.00 atm (point B). Then it is allowed to expand isothermally to 1.00 atm (point C) and at last compressed isobarically to its original state, (a) Find the number of moles in the sample.
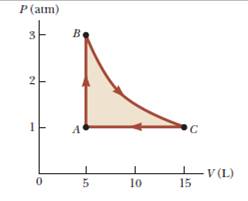
Find (b) the temperature at point B, (c) the temperature at point C, and (d) the volume at point C. (e) Now consider the processes A → B, B→ C, and C → A. Describe how to carry out each process experimentally, (f) Find Q, W, and ΔEint for each of the processes, (g) For the whole cycle A→ B→ C→ A, find Q, W, and ΔEint.
(a)
The number of moles in the sample.
Answer to Problem 21.65AP
The number of moles in the sample is
Explanation of Solution
Given info: The volume of the monatomic ideal gas is
The number of moles in the ideal gas equation is,
Here,
The value of the ideal gas constant is
Substitute
Conclusion:
Therefore, the number of moles in the sample is
(b)
The temperature at point
Answer to Problem 21.65AP
The temperature at point
Explanation of Solution
Given info: The volume of the monatomic ideal gas is
In the process from point
The expression for the process from point
Here,
Substitute
Conclusion:
Therefore, the temperature at point
(c)
The temperature at point
Answer to Problem 21.65AP
The temperature at point
Explanation of Solution
Given info: The volume of the monatomic ideal gas is
In the process from point
So, the temperature at point
Conclusion:
Therefore, the temperature at point
(d)
The volume at point
Answer to Problem 21.65AP
The volume at point
Explanation of Solution
Given info: The volume of the monatomic ideal gas is
In the process from point
The expression for the process from point
Here,
Substitute
Conclusion:
Therefore, the volume at point
(e)
The experimental methods to carry out the process
Answer to Problem 21.65AP
The experimental method to carry out the process
Explanation of Solution
Given info: The volume of the monatomic ideal gas is
In the process from point
The volume does not change. The temperature varies from
In the process from point
The temperature does not change. The pressure varies from
In the process from point
The pressure does not change. The temperature varies from
Conclusion:
Therefore, the experimental method to carry out the process
(f)
The heat
Answer to Problem 21.65AP
The heat
Explanation of Solution
Given info: The volume of the monatomic ideal gas is
For the process from point
The volume of gas does not change.
The work done is,
The change in internal energy is equal to the heat.
The expression for the change in internal energy is,
Here,
Substitute
Substitute
Thus, change in internal energy in process from point
For the process from point
The temperature does not change.
The change in internal energy is,
The expression of the work done is,
Substitute
Thus the change in internal energy in process from point
For the process from point
The formula of work done is,
Substitute
Thus, the work done for the point
The formula for the change in kinetic energy is,
Substitute
The heat obtain in this process is,
Conclusion:
Therefore, the heat
(g)
The heat
Answer to Problem 21.65AP
For the whole cycle
Explanation of Solution
Given info: The volume of the monatomic ideal gas is
The expression for the heat in complete cycle is,
Substitute
Thus, the heat in cycle is
The expression for the work done in complete cycle is,
Substitute
Thus, the total work done is
As the process is cyclic, the change in internal energy will be zero.
Conclusion:
Therefore, For the whole cycle
Want to see more full solutions like this?
Chapter 21 Solutions
Physics for Scientists and Engineers
- Considering the cross-sectional area shown in Fig.2: 1. Determine the coordinate y of the centroid G (0, ỹ). 2. Determine the moment of inertia (I). 3. Determine the moment of inertia (Ir) (with r passing through G and r//x (// parallel). 4 cm 28 cm G3+ G 4 cm y 12 cm 4 cm 24 cm xarrow_forwardI need help understanding 7.arrow_forwardThe stress-strain diagram for a steel alloy is given in fig. 3. Determine the modulus of elasticity (E). σ (ksi) 40 30 20 10 0 0 0.0005 0.001 0.0015 0.002 0.0025 0.0030.0035 Earrow_forward
- A Van de Graff generator, if the metal sphere on the Van de Graff has a charge of 0.14 Coulombs and the person has a mass of 62 kg, how much excess charge would the person need in order to levitate at a distance 25 cm from the center of the charged metal sphere? Assume you can treat both the person and the metal sphere as point charges a distance 25 cm from each other using Coulomb's Law to calculate the electrical force. Give your answer as the number of Coulombsarrow_forwardPlease help me answer the following question. I am having trouble understanding the directions of the things the question is asking for. Please include a detailed explanation and possibly drawings of the directions of Bsource, Binduced, and Iinduced.arrow_forward43. A mass må undergoes circular motion of radius R on a hori- zontal frictionless table, con- nected by a massless string through a hole in the table to a second mass m² (Fig. 5.33). If m₂ is stationary, find expres- sions for (a) the string tension and (b) the period of the circu- lar motion. m2 R m₁ FIGURE 5.33 Problem 43arrow_forward
- CH 70. A block is projected up an incline at angle 0. It returns to its initial position with half its initial speed. Show that the coefficient of ki- netic friction is μk = tano.arrow_forwardPassage Problems A spiral is an ice-skating position in which the skater glides on one foot with the other foot held above hip level. It's a required element in women's singles figure-skating competition and is related to the arabesque performed in ballet. Figure 5.40 shows Canadian skater Kaetlyn Osmond executing a spiral during her medal-winning perfor- mance at the 2018 Winter Olympics in Gangneung, South Korea. 77. From the photo, you can conclude that the skater is a. executing a turn to her left. b. executing a turn to her right. c. moving in a straight line out of the page. 78. The net force on the skater a. points to her left. b. points to her right. c. is zero. 79. If the skater were to execute the same maneuver but at higher speed, the tilt evident in the photo would be a. less. b. greater. c. unchanged. FIGURE 5.40 Passage Problems 77-80 80. The tilt angle 0 that the skater's body makes with the vertical is given ap- proximately by 0 = tan¯¹(0.5). From this you can conclude…arrow_forwardFrictionless surfarrow_forward
- 71. A 2.1-kg mass is connected to a spring with spring constant 72 k = 150 N/m and unstretched length 18 cm. The two are mounted on a frictionless air table, with the free end of the spring attached to a frictionless pivot. The mass is set into circular mo- tion at 1.4 m/s. Find the radius of its path. cor moving at 77 km/h negotiat CH —what's the minimum icient of frictioarrow_forward12. Two forces act on a 3.1-kg mass that undergoes acceleration = 0.91 0.27 m/s². If one force is -1.2î – 2.5ĵ N, what's the other?arrow_forward36. Example 5.7: You whirl a bucket of water around in a vertical circle of radius 1.22 m. What minimum speed at the top of the circle will keep the water in the bucket?arrow_forward
 Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning





