
Concept explainers
Draw structures for the following:
- a. guanosine-5′-monophosphate
- b. cytidine-5′-diphosphate
- c. dAMP
- d. thymidine
(a)
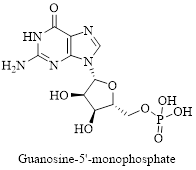
Interpretation: Structure for the given compound guanosine-5’-monophosphate has to be drawn.
Concept introduction:
In chemistry, structure is a form of the molecular formula that indicates the arrangement of atoms within a molecule by connecting atoms with a line or lines to represent a chemical bond.
Explanation of Solution
The given compound is guanosine-5’-monophosphate and it structure is drawn below,
(b)
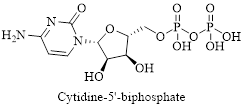
Interpretation: Structure for the given compound cytidine-5’-diphosphate has to be drawn.
Concept introduction:
In chemistry, structure is a form of the molecular formula that indicates the arrangement of atoms within a molecule by connecting atoms with a line or lines to represent a chemical bond.
Explanation of Solution
The given compound is cytidine-5’-diphosphate, its structure can be drawn as follows,
(c)
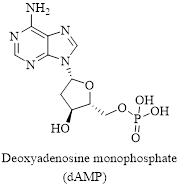
Interpretation: Structure for the given compound dAMP has to be drawn.
Concept introduction:
In chemistry, structure is a form of the molecular formula that indicates the arrangement of atoms within a molecule by connecting atoms with a line or lines to represent a chemical bond.
Explanation of Solution
The given compound is dAMP and it is the abbreviation for the compound named Deoxyadenosine monophosphate.
The structure of the compound can be drawn as shown below,
(d)
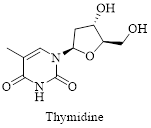
Interpretation: Structure for the given compound thymidine has to be drawn.
Concept introduction:
In chemistry, structure is a form of the molecular formula that indicates the arrangement of atoms within a molecule by connecting atoms with a line or lines to represent a chemical bond.
Explanation of Solution
The given compound is thymidine and its structure can be drawn as shown below,
Want to see more full solutions like this?
Chapter 21 Solutions
Essential Organic Chemistry (3rd Edition)
- Name the following molecules with IUpacarrow_forwardWhat is the molecular orbital for cyclopropenyl anion and is it aromatic, antiaromatic or nonaromatic?arrow_forwardUsing the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forward
- How to get the predicted product of this reaction belowarrow_forwardPlease help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forwardWrite the Esterification reaction mechanism for acetic acid, and one propanol to make propanol ethanoate (molecule that gives peas its odor in flavor)arrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





