
Concept explainers
A Boron and hydrogen form an extensive family of compounds, and the diagram below shows how they are related by reaction.
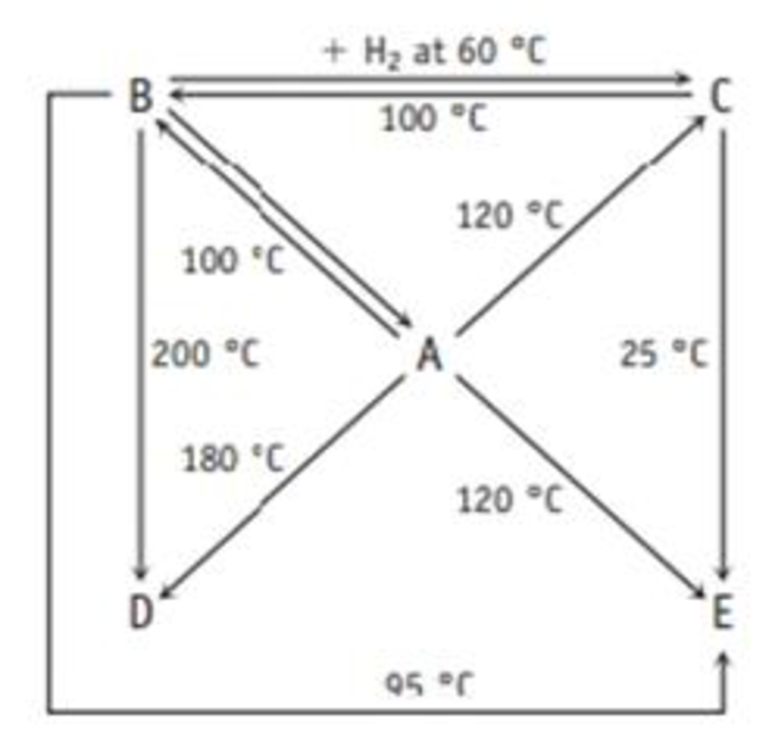
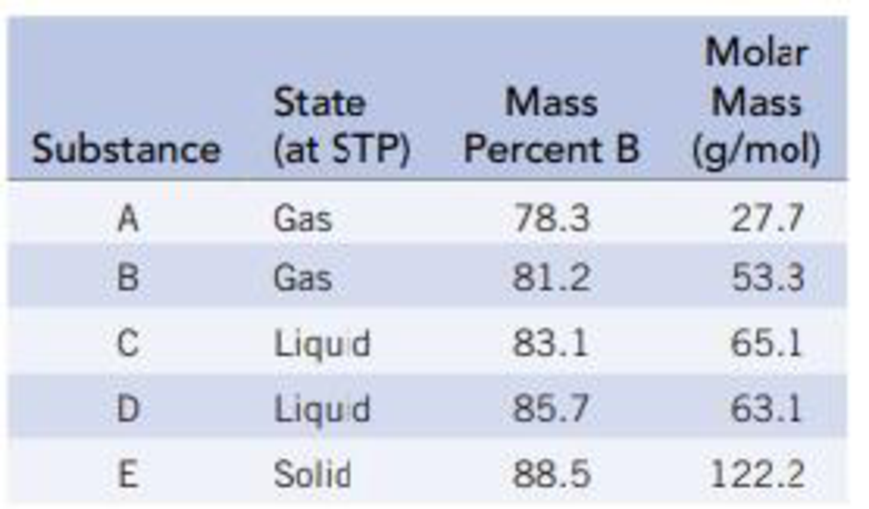
The following table gives the weight percent of boron in each of the compounds. Derive the empirical and molecular formulas of compounds A-E.
Interpretation: To determine the empirical and molecular formula of given compounds A-E.
Concept introduction:
The empirical formula of a chemical compound is the simplest positive integer ratio of atoms present in a compound.
A molecular formula shows the total number of atoms in a molecule but not their structural arrangement.
Answer to Problem 107GQ
The empirical formula of compound A is
The empirical formula of compound B is
The empirical formula of compound C is
The empirical formula of compound D is
The empirical formula of compound E is
Explanation of Solution
Boron and hydrogen form an extensive family of compounds. Substance A-E contains boron and hydrogen atoms.
The empirical and molecular formula of given compounds A-E is calculated below.
Given:
Substance A is a gaseous compound contains
The empirical formula of substance A is calculated as,
Convert the mass of boron and hydrogen into moles using molar mass of boron and hydrogen respectively.
Divide each mole value by the smallest number of moles calculated. Round off to the nearest whole number.
Thus, the empirical formula of compound A is
The empirical formula molar mass of compound A is
Divide the molecular formula mass by the empirical formula mass,
Multiply each of the subscripts within the empirical formula of substance A by the number calculated above.
Thus, the molecular formula of substance A is
Substance B is a gaseous compound contains
The empirical formula of substance B is calculated as,
Convert the mass of boron and hydrogen into moles using molar mass of boron and hydrogen respectively.
Divide each mole value by the smallest number of moles calculated. Round off to the nearest whole number.
Thus, the empirical formula of compound B is
The empirical formula molar mass of compound B is
Divide the molecular formula mass by the empirical formula mass,
Multiply each of the subscripts within the empirical formula of substance B by the number calculated above.
Thus, the molecular formula of substance B is
Substance C is a liquid compound contains
The empirical formula of substance C is calculated as,
Convert the mass of boron and hydrogen into moles using molar mass of boron and hydrogen respectively.
Divide each mole value by the smallest number of moles calculated. Round off to the nearest whole number.
Thus, the empirical formula of compound C is
The empirical formula molar mass of compound A is
Divide the molecular formula mass by the empirical formula mass,
Multiply each of the subscripts within the empirical formula of substance C by the number calculated above.
Thus, the molecular formula of substance C is
Substance D is a liquid compound contains
The empirical formula of substance D is calculated as,
Convert the mass of boron and hydrogen into moles using molar mass of boron and hydrogen respectively.
Divide each mole value by the smallest number of moles calculated. Round off to the nearest whole number.
Thus, the empirical formula of compound D is
The empirical formula molar mass of compound A is
Divide the molecular formula mass by the empirical formula mass,
Multiply each of the subscripts within the empirical formula of substance D by the number calculated above.
Thus, the molecular formula of substance D is
Substance E is a solid compound contains
The empirical formula of substance E is calculated as,
Convert the mass of boron and hydrogen into moles using molar mass of boron and hydrogen respectively.
Divide each mole value by the smallest number of moles calculated. Round off to the nearest whole number.
Thus, the empirical formula of compound E is
The empirical formula molar mass of compound E is
Divide the molecular formula mass by the empirical formula mass,
Multiply each of the subscripts within the empirical formula of substance E by the number calculated above.
Thus, the molecular formula of substance E is
The empirical formula of compound A is
The empirical formula of compound B is
The empirical formula of compound C is
The empirical formula of compound D is
The empirical formula of compound E is
Want to see more full solutions like this?
Chapter 21 Solutions
OWLv2 6-Months Printed Access Card for Kotz/Treichel/Townsend's Chemistry & Chemical Reactivity, 9th, 9th Edition
- Q1: a) Arrange the compounds in order of decreasing pKa, highest first. ОН ΟΗ ῸΗ дон ОН ОН CI Brarrow_forward(4 pts - 2 pts each part) A route that can be taken to prepare a hydrophobic (water-repellent) aerogel is to start with trichloromethylsilane, CH3SiCl3 as the silicon source. a. What is the chemical reaction that this undergoes to form a product with Si-OH groups? Write as complete of a chemical equation as you can. CI CI-SI-CH3 CI b. The formation of a byproduct is what drives this reaction - what is the byproduct (if you didn't already answer it in part (a)) and how/why does it form?arrow_forwardb) Circle the substrate that would not efficiently generate a Grignard reagent upon reaction with Mg in ether. CI Br ד c) Circle the Grignard reagents that contain incompatible functional groups. MgBr HO MgBr MgBr MgBr MgBr HO MgBrarrow_forward
- Q2: Predict all organic product(s), including stereoisomers when applicable. PCC OH a) CH2Cl2 Page 2 of 5 Chem 0310 Organic Chemistry 1 HW Problem Sets b) .OH Na2Cr2O7, H+ OH PCC CH2Cl2 c) OHarrow_forwardd) Circle the substrates that will give an achiral product after a Grignard reaction with CH3MgBr. Harrow_forwardQ4: Predict the organic products for the following reactions. Then draw curved arrow electron- pushing mechanism for the reactions. a) NaBH4 EtOH Page 4 of 5 Chem 0310 Organic Chemistry 1 HW Problem Sets b) 요 1. Et₂O H MgBr 2. H+, H₂Oarrow_forward
- Would Si(CH3)3F react with AgCl? If so, write out the balanced chemical equation. If not,explain why no reaction would take place.arrow_forwardNH3 reacts with boron halides (BX3 where X = F, Cl, Br, or I) to form H3N-BX3 complexes.Which of these complexes will have the strongest N-B bond? Justify your answerarrow_forward3Helparrow_forward
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





