
ORGANIC CHEMISTRY W/OWL
9th Edition
ISBN: 9781305717527
Author: McMurry
Publisher: CENGAGE C
expand_more
expand_more
format_list_bulleted
Question
Chapter 20.SE, Problem 41AP
Interpretation Introduction
Interpretation:
The pKa of uric acid is 5.61. The percent dissociation of uric acid in urine at a pH=6.0 is to be calculated. Why uric acid is acidic even though it does not have a COOH group is to be explained.
Concept introduction:
The Henderson-Hasselbalch equation is
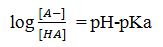
where, [A-] is the concentration of the dissociated uric acid and [HA] is the concentration of the undissociated uric acid.
A compound will be acidic and donate a proton, if the conjugate base obtained is stabilized by resonance.
To calculate:
The percent dissociation of uric acid in urine at a pH=6.0 if its pKa is 5.61 and to explain why uric acid is acidic even though it does not have a COOH group.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Please explain how to calculate the pH.
I'm having trouble with converting lewis diagrams into VSEPR diagrams. I currently have this example of C2BrCl3 which I want to turn into a lewis structure, but I'm not sure what steps I need to do in order to do so. I have the table written down, however, there's two central atoms so what would I do? There seems to be 4 electron domains on the carbon atom and no lone pairs so it would seem like this shape would be tetrahedral. Here's what I have now. Thanks!
We discussed the solid phase resin using in peptide synthesis. Provide a mechanism, for its formation. DRAW THE MECHANISM.
Chapter 20 Solutions
ORGANIC CHEMISTRY W/OWL
Ch. 20.1 - Give IUPAC names for the following compounds:Ch. 20.1 - Draw structures corresponding to the following...Ch. 20.2 - Prob. 3PCh. 20.2 - The Ka for dichloroacetic acid is 3.32 Ă— 10-2....Ch. 20.3 - Calculate the percentages of dissociated and...Ch. 20.4 - Which would you expect to be a stronger acid, the...Ch. 20.4 - Dicarboxylic acids have two dissociation...Ch. 20.4 - The pKa of p-cyclopropylbenzoic acid is 4.45. Is...Ch. 20.4 - Prob. 9PCh. 20.5 - Prob. 10P
Ch. 20.6 - Prob. 11PCh. 20.6 - How might you carry out the following...Ch. 20.7 - Prob. 13PCh. 20.7 - Prob. 14PCh. 20.8 - Cyclopentanecarboxylic acid and...Ch. 20.8 - Prob. 16PCh. 20.SE - Prob. 17VCCh. 20.SE - Prob. 18VCCh. 20.SE - The following carboxylic acid can’t be prepared...Ch. 20.SE - Electrostatic potential maps of anisole and...Ch. 20.SE - Predict the product(s) and provide the mechanism...Ch. 20.SE - Predict the product(s) and provide the mechanism...Ch. 20.SE - Prob. 23MPCh. 20.SE - Predict the product(s) and provide the complete...Ch. 20.SE - Acid-catalyzed hydrolysis of a nitrile to give a...Ch. 20.SE - Prob. 26MPCh. 20.SE - Naturally occurring compounds called cyanogenic...Ch. 20.SE - 2-Bromo-6, 6-dimethylcyclohexanone gives 2,...Ch. 20.SE - Naturally occurring compounds called terpenoids,...Ch. 20.SE - In the Ritter reaction, an alkene reacts with a...Ch. 20.SE - Give IUPAC names for the following compounds:Ch. 20.SE - Prob. 32APCh. 20.SE - Prob. 33APCh. 20.SE - Prob. 34APCh. 20.SE - Prob. 35APCh. 20.SE - Prob. 36APCh. 20.SE - Prob. 37APCh. 20.SE - Prob. 38APCh. 20.SE - Calculate the Ka's for the following acids: (a)...Ch. 20.SE - Thioglycolic acid, HSCH2CO2H, a substance used in...Ch. 20.SE - Prob. 41APCh. 20.SE - Prob. 42APCh. 20.SE - How could you convert butanoic acid into the...Ch. 20.SE - How could you convert each of the following...Ch. 20.SE - How could you convert butanenitrile into the...Ch. 20.SE - How would you prepare the following compounds from...Ch. 20.SE - Prob. 47APCh. 20.SE - Using 13CO2 as your only source of labeled carbon,...Ch. 20.SE - Prob. 49APCh. 20.SE - Which method-Grignard carboxylation or nitrile...Ch. 20.SE - Prob. 51APCh. 20.SE - Prob. 52APCh. 20.SE - Propose a structure for a compound C6H12O2 that...Ch. 20.SE - Prob. 54APCh. 20.SE - How would you use NMR (either 13C or 1H) to...Ch. 20.SE - Prob. 56APCh. 20.SE - A chemist in need of 2,2-dimethylpentanoic acid...Ch. 20.SE - Prob. 58APCh. 20.SE - Prob. 59APCh. 20.SE - Prob. 60APCh. 20.SE - Prob. 61APCh. 20.SE - Prob. 62APCh. 20.SE - Prob. 63APCh. 20.SE - The following pKa values have been measured....Ch. 20.SE - Identify the missing reagents a-f in the following...Ch. 20.SE - Propose a structure for a compound, C4H7N, that...Ch. 20.SE - Prob. 67APCh. 20.SE - The 1H and 13C NMR spectra below belong to a...Ch. 20.SE - Propose structures for carboxylic acids that show...Ch. 20.SE - Carboxylic acids having a second carbonyl group...
Knowledge Booster
Similar questions
- (a) 21.8 Name the following compounds. & (b) Br (e) O₂N. (h) H (c) Br (d) NH2 ☑N Br H ہیں Ph (g) OMe бл .0-0.e 21.9 Draw a structural formula for each compound. (a) 2,3-Dinitrotoluene (c) Diphenylmethanol (e) p-Nitroaniline (b) 3-Propylanisole (d) m-Propylphenol (f) Pentabromobenzenearrow_forwardIs this the major product of this reaction?arrow_forwardPlease helparrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning