
EP ESSENTIAL ORG.CHEM.-MOD.MASTERING
3rd Edition
ISBN: 9780133858501
Author: Bruice
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 20.6, Problem 11P
Interpretation Introduction
Interpretation:
The isoprene units has to be marked in lycopene and
Concept Introduction:
Terpenes are made by joining five-carbon units, usually in a head to tail-fashion.
Monoterpenes are those terpenes with two isoprene units-have 10 carbons, sesquiterpenes have 15 carbons, diterpenes have 20 carbons, triterpenes have 30 carbons and tetraterpenes have 40 carbons.
Isopentenyl pyrophosphate (isoprene) is the five-carbon compound used for the biosynthesis of terpenes.
Isoprene unit:
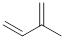
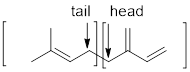
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the major product of the Claisen condensation reaction between two molecules of this ester. Ignore
inorganic byproducts.
Incorrect, 5 attempts remaining
1. NaOCH3/CH3OH
2. Acidic workup
Select to Draw
O
Incorrect, 5 attempts remaining
The total number of carbons in the parent chain is incorrect. Review the reaction conditions including starting materials and/or
intermediate structures and recount the number of carbon atoms in the parent chain of your structure.
OK
Using a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40.
a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L)
b) Calculate the molar absorptivity, a, of the 1645 cm-1 band
c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c
d) Calculate x for the 1645 cm-1 band
Convert 1.38 eV into wavelength (nm) and wavenumber (cm-1) (c = 2.998 x 108 m/s; h = 6.626 x 10-34 J*s).
Chapter 20 Solutions
EP ESSENTIAL ORG.CHEM.-MOD.MASTERING
Ch. 20.1 - Prob. 1PCh. 20.2 - Prob. 2PCh. 20.2 - Prob. 3PCh. 20.2 - Draw the structure of an optically active fat...Ch. 20.4 - Prob. 6PCh. 20.4 - Prob. 7PCh. 20.4 - The membrane phospholipids in deer have a higher...Ch. 20.4 - Prob. 9PCh. 20.6 - Prob. 10PCh. 20.6 - Prob. 11P
Ch. 20.6 - Prob. 12PCh. 20.7 - Propose a mechanism for the biosynthesis of...Ch. 20.7 - Prob. 14PCh. 20.8 - Draw the individual 1,2-hydride and 1,2-methyl...Ch. 20.9 - Prob. 16PCh. 20 - Prob. 17PCh. 20 - Prob. 18PCh. 20 - Cardiolipins are found in heart muscles. Draw the...Ch. 20 - Prob. 20PCh. 20 - 5-Androstene-3,17-dione is isomerized to...Ch. 20 - Prob. 22PCh. 20 - Prob. 23PCh. 20 - Prob. 24PCh. 20 - Eudesmol is a sesquiterpene found in eucalyptus....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Can you help me understand the CBC method on metal bridging by looking at this problem?arrow_forwardA partir de Aluminio y Co(NO3)2ꞏ6H2O, indicar las reacciones a realizar para obtener Azul de Thenard (Al2CoO4).arrow_forwardTo obtain Thenard Blue (Al2CoO4), the following reaction is correct (performed in an oven):Al(OH)3 + Co(OH)2 → Al2CoO4 + 4 H2Oarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning