
Interpretation: The diene and the dienophile that might be used to prepare the given racemic Diels-Alder adduct have to be found.
Concept Introduction:
Diels-Alder reaction:
It is the reaction of conjugated dienes with double or triple bonded compounds which are known as “dienophiles”. The reaction is a
Example:
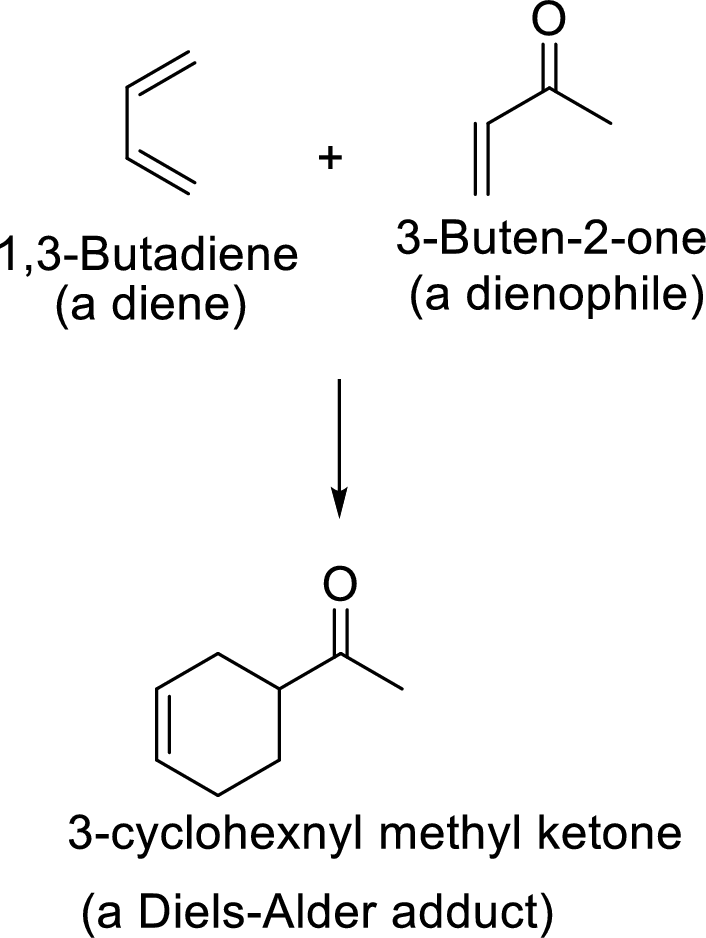
Diels-Alder reaction to form bicyclic system:
The Diels-Alder adduct formed in the Diels-Alder reaction can also be a bicyclic system which will be obtained when cylopentadiene is used as the diene as shown here:
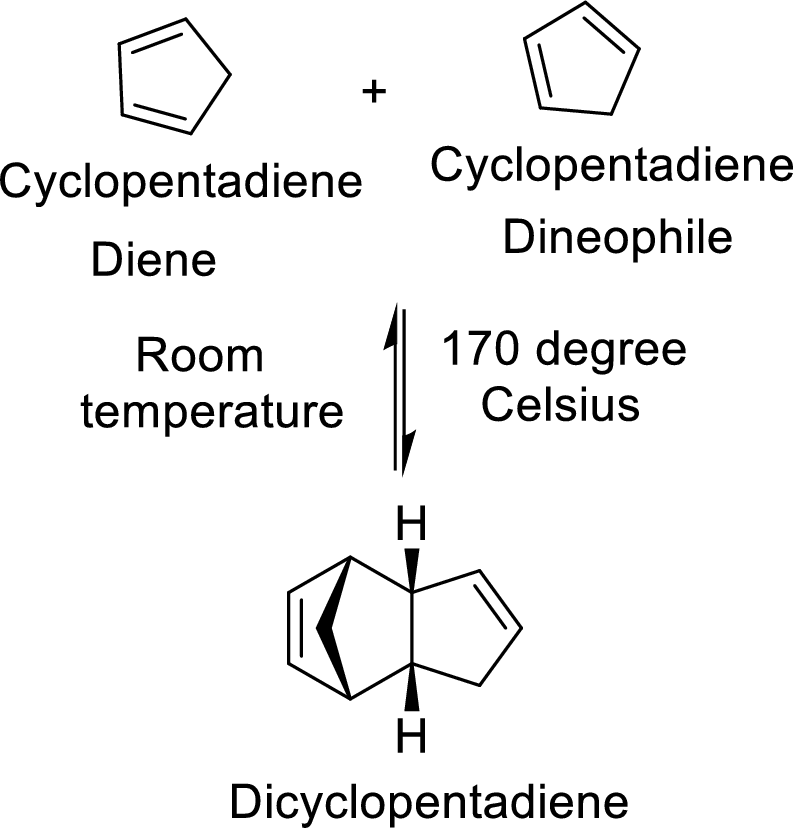
In this reaction, the cylopentadiene acts as both diene and dienophile and formed a bicyclic system. When it is heated to
Racemic mixture in Diels-alder reaction:
In the Diels-alder reaction, the formation of two new sigma bonds results in the formation of two new chiral centres that are enantiomers to each other. So, the Diels-alder adduct is the mixture of two enantiomers and therefore it is being a racemic Diels-alder adduct.
Trending nowThis is a popular solution!

Chapter 20 Solutions
OWL V2 with MindTap Reader and Student Solutions Manual eBook for Brown/Iverson/Anslyn/Foote's Organic Chemistry, 8th Edition
- K Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward7 Comment on the general features of the predicted (extremely simplified) ¹H- NMR spectrum of lycopene that is provided below. 00 6 57 PPM 3 2 1 0arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

