
EBK GENERAL CHEMISTRY
11th Edition
ISBN: 9780133400588
Author: Bissonnette
Publisher: VST
expand_more
expand_more
format_list_bulleted
Question
Chapter 20, Problem 57E
Interpretation Introduction
(a)
Interpretation:
For the reactions at about room temperature, the approximate activation energy is to be determined for the given statement to be true.
Concept introduction:
- Arrhenius equation relates the rate constant (k) of the reaction to its absolute temperature as
- At two different temperatures, Arrhenius equation can be modified as
Where
A − Pre-exponential factor
Ea- Activation energy
T- Absolute temperature in Kelvins
R- Universal gas constant
Interpretation Introduction
(b)
Interpretation:
Whether the thumb rule is applied or not to the following reaction profile at room temperature for reaction-
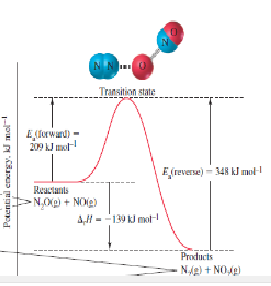
Concept introduction:
- Arrhenius equation relates the rate constant (k) of the reaction to its absolute temperature as
- At two different temperatures, Arrhenius equation can be modified as
Where
A − Pre-exponential factor
Ea- Activation energy
T- Absolute temperature in Kelvins
R- Universal gas constant
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
6. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic.
B22+
B22+, B2, C22, B22 and N22+
Molecular Orbital Diagram
B2
C22-
B22-
N22+
Which molecule is paramagnetic?
3. Put the following species in order of increasing bond length by using molecular orbital diagrams and
calculating their bond orders: F2, F2, F2+
Molecular Orbital Diagram
F2
F2
F2+
Bond Order
Shortest bond:
Longest bond
3. Put the following species in order of increasing bond length by using molecular orbital diagrams and
calculating their bond orders: F2, F2, F2+
Molecular Orbital Diagram
F2
F2
F2+
Bond Order
Chapter 20 Solutions
EBK GENERAL CHEMISTRY
Ch. 20 - In the reaction 2A+BC+3D , reactant A is found to...Ch. 20 - From Figure 20-2 estimate the rate of reaction at...Ch. 20 - In the reaction A products, [A] is found to be...Ch. 20 - In the reaction A products, at t = 0. [A]= 0.1565...Ch. 20 - In the reaction A products. 4.40 min after the...Ch. 20 - Refer to Experiment 2 of Table 20.3 and to...Ch. 20 - For the reaction A+2BC ,the rate of reaction is...Ch. 20 - If the rate of reaction (20.3) is 5.7104 M s-1 ,...Ch. 20 - In the reaction A(g)B(g)+C(g) , the totalpressure...Ch. 20 - At 65C , the half-life for the first-order...
Ch. 20 - The initial rate of the reaction A+BC+D is...Ch. 20 - For the reaction A+BC+D , the following initial...Ch. 20 - Prob. 13ECh. 20 - The following data are obtained for the initial...Ch. 20 - One of the following statements is true and the...Ch. 20 - One of the following statements true and the other...Ch. 20 - The first-order reaction A products has t1/2=180...Ch. 20 - The reaction A products is first order in A....Ch. 20 - The reaction A products is first order A. a. If...Ch. 20 - In the first-order reaction A products, [A] =...Ch. 20 - In the first-order reaction A products, it found...Ch. 20 - The half-life of me radioactive isotope...Ch. 20 - Acetoacetic acid, CH2COOH2COOH , a reagent in...Ch. 20 - The following first-order reaction occurs in...Ch. 20 - For the reaction A- products, the following data...Ch. 20 - The decomposition of dimethyl ether at 504C is (...Ch. 20 - [Hint: There are several of arrivivg at answer for...Ch. 20 - [Hint: There are several of arrivivg at answer for...Ch. 20 - Prob. 29ECh. 20 - Prob. 30ECh. 20 - Prob. 31ECh. 20 - Prob. 32ECh. 20 - [Hint: There are several of arrivivg at answer for...Ch. 20 - [Hint: There are several ways of arrivivig at...Ch. 20 - Prob. 35ECh. 20 - Prob. 36ECh. 20 - For the reaction A products, the following data...Ch. 20 - Prob. 38ECh. 20 - For the reaction A products, the data tabulated...Ch. 20 - For the reaction A2B+C , the following data are...Ch. 20 - In three different experiments, the following...Ch. 20 - Ammonia decomposes on the surface of a hot...Ch. 20 - Prob. 43ECh. 20 - Consider three hypothetical reactions A — products...Ch. 20 - Prob. 45ECh. 20 - If even tiny sped is introduced into a mixture of...Ch. 20 - For me reversible reaction A+BC+D , the enthalpy...Ch. 20 - Prob. 48ECh. 20 - By inspection of the reaction profile for the...Ch. 20 - By inspection of the reaction profile for the...Ch. 20 - The rate constant for the reaction...Ch. 20 - At what temperature will the rate constant for the...Ch. 20 - Prob. 53ECh. 20 - The reaction C2H5+OHC2H5OH+I was studied in an...Ch. 20 - The first-order reaction A products has a...Ch. 20 - For the first-order reaction N2O4(g)2NO2+12O2g...Ch. 20 - Prob. 57ECh. 20 - Concerning the rule of thumb stated r Exercise 57,...Ch. 20 - The following statements about catalysis are not...Ch. 20 - Prob. 60ECh. 20 - What are the similarities and differences between...Ch. 20 - Certain gas-phase reactions on a heterogeneous...Ch. 20 - The graph show s the effect of enzyme...Ch. 20 - The graph shows the effect of temperature on...Ch. 20 - Prob. 65ECh. 20 - Prob. 66ECh. 20 - The reaction 2NO+2H2N2+2H2O is second order m [NO]...Ch. 20 - The mechanism proposed for me reaction of H2(g)...Ch. 20 - The reaction 2NO+Cl22NOCl has rate law: rate of...Ch. 20 - A simplified rate law 1o the reaction 2O2(g)3O2(g)...Ch. 20 - Prob. 71ECh. 20 - One proposed meachanism for the condensation of...Ch. 20 - Suppose that the reaction r Example 20-8 is first...Ch. 20 - [A]t as a function of time for the reaction A —...Ch. 20 - Exactly 300 s after decomposition of H2O2(aq)...Ch. 20 - Use the method of Exercise 75 to determine the...Ch. 20 - Prob. 77IAECh. 20 - Prob. 78IAECh. 20 - Hydroxide ion is involved in the mechanism of the...Ch. 20 - The half-life for the first-order decomposition of...Ch. 20 - The decomposition of ethylene oxide at 690 K is...Ch. 20 - Prob. 82IAECh. 20 - The following data are for the reaction 2 A + B ...Ch. 20 - Prob. 84IAECh. 20 - Prob. 85IAECh. 20 - Prob. 86IAECh. 20 - The following three-step mechanism has been...Ch. 20 - Prob. 88IAECh. 20 - Prob. 89IAECh. 20 - Prob. 90IAECh. 20 - Prob. 91IAECh. 20 - Prob. 92IAECh. 20 - Prob. 93IAECh. 20 - You want to test the following proposed mechanism...Ch. 20 - Prob. 95IAECh. 20 - Benzenediazonium chloride decomposes by a...Ch. 20 - The object is to study the kinetics of the...Ch. 20 - Prob. 98SAECh. 20 - Prob. 99SAECh. 20 - Explain the important distinctions between each...Ch. 20 - Prob. 101SAECh. 20 - A first-order reaction A — products, has a...Ch. 20 - Prob. 103SAECh. 20 - Prob. 104SAECh. 20 - The rate of a chemical reaction generally...Ch. 20 - For the reaction A+B2C, which proceeds by a...Ch. 20 - Prob. 107SAECh. 20 - Prob. 108SAECh. 20 - Prob. 109SAECh. 20 - For me reaction A products the following data are...Ch. 20 - For the reaction A+2BC+D , the rate law is rate...Ch. 20 - Prob. 112SAECh. 20 - If the plot of the reactant concentration versus...Ch. 20 - Prob. 114SAECh. 20 - Prob. 115SAE
Knowledge Booster
Similar questions
- 4. The superoxide ion, Oz, plays an important role in the ageing processes that take place in organisms. Judge whether Oz is likely to have larger or smaller dissociation energy than 02. Molecular Orbital Diagram 02 02 Does O2 have larger or smaller dissociation energy?: Bond Orderarrow_forward1. How many molecular orbitals can be built from the valence shell orbitals in O2?arrow_forwardSho reaction mechanism. Don't give Ai generated solutionarrow_forward
- Is this aromatic, antiaromatic, or nonaromatic?arrow_forwardOn what basis are Na and Nb ranked against each other?arrow_forwardStep 1: add a curved arrow. Select Draw Templates More / " C H Br 0 Br : :o: Erase H H H H Q2Q Step 2: Draw the intermediates and a curved arrow. Select Draw Templates More MacBook Air / " C H Br 0 9 Q Erase 2Qarrow_forward
- O Macmillan Learning Question 23 of 26 > Stacked Step 7: Check your work. Does your synthesis strategy give a substitution reaction with the expected regiochemistry and stereochemistry? Draw the expected product of the forward reaction. - - CN DMF MacBook Air Clearly show stereochemistry. Questionarrow_forwardNH2 1. CH3–MgCl 2. H3O+ ? As the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will make a molecule with a new C - C bond as its major product: If this reaction will work, draw the major organic product or products you would expect in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If the major products of this reaction won't have a new C - C bond, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. This reaction will not make a product with a new C - C bond. Х ☐: Carrow_forwardPredict the major products of this organic reaction. If there will be no major products, check the box under the drawing area instead. No reaction. : + Х è OH K Cr O 2 27 2 4' 2 Click and drag to start drawing a structure.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
