
Concept explainers
(a)
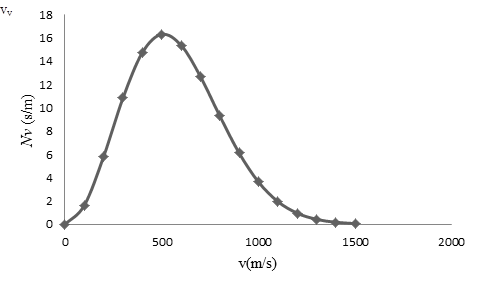
To draw: The graph of the Maxwell speed distribution function versus speed with points at speed intervals of
(a)
Answer to Problem 38AP
The of the Maxwell speed distribution function versus speed with points at speed intervals of
Explanation of Solution
Introduction:
The Maxwell distribution curve is the graph between the distribution of speed and the change in speed or speed interval.
Given info: The number of molecules of oxygen in vessel is
Write the expression of Maxwell’s speed distribution function.
Here,
The mass of the molecules of oxygen is,
Here,
The molecular mass of the oxygen molecules in
Substitute
Substitute
Substitute the values of
|
|
|
| 0 | 0 |
| 100 | 1.64 |
| 200 | 5.86 |
| 300 | 10.88 |
| 400 | 14.78 |
| 500 | 16.33 |
| 600 | 15.39 |
| 700 | 12.7 |
| 800 | 9.31 |
| 900 | 6.13 |
| 1000 | 3.64 |
| 1100 | 1.961 |
| 1200 | 0.96 |
| 1300 | 0.43 |
| 1400 | 0.18 |
| 1500 | 0.07 |
On the basis of the table, a graph is plotted below;
(b)
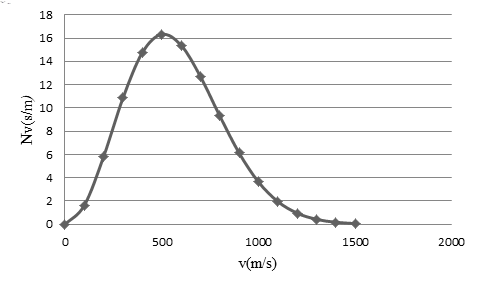
The most probable speed from the graph.
(b)
Answer to Problem 38AP
The most probable speed is
Explanation of Solution
Given info: The number of molecules of oxygen in vessel is
The most probable speed occurs where
Conclusion:
Therefore, the most probable speed is
(c)
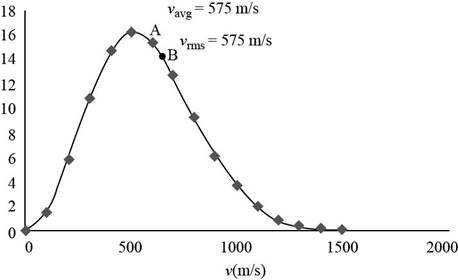
The average and rms speeds for the molecules and label these points on the graph.
(c)
Answer to Problem 38AP
The average and rms speeds for the molecules is
Explanation of Solution
Given info: The number of molecules of oxygen in vessel is
Write the expression of average velocity.
The mass of the molecules of oxygen is,
Substitute
The molecular mass of the oxygen molecules in
Substitute
Thus, the average speed is
Write the expression of rms velocity.
Substitute
Substitute
Thus, the rms velocity of the oxygen molecules is
The graph of Maxwell’s curve is shown below;
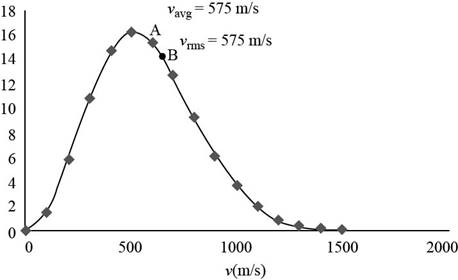
Figure (1)
The point
Conclusion:
Therefore, the average and rms speeds for the molecules is
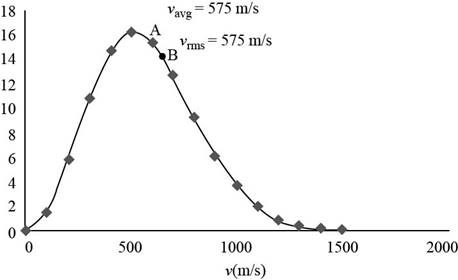
(d)
The fraction of molecules with the speed in the range of
(d)
Answer to Problem 38AP
The fraction of molecules with the speed in the range of
Explanation of Solution
Given info: The number of molecules of oxygen in vessel is
The figure given below shows the Maxwell’s curve,
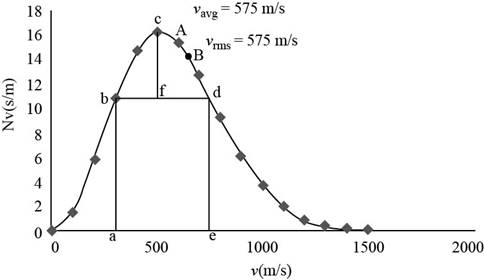
Figure 2
Write the expression of the fraction of molecules with the speed range from
Substitute
Conclusion:
Therefore, the fraction of molecules with the speed in the range of
Want to see more full solutions like this?
Chapter 20 Solutions
Bundle: Physics For Scientists And Engineers With Modern Physics, Loose-leaf Version, 10th + Webassign Printed Access Card For Serway/jewett's Physics For Scientists And Engineers, 10th, Single-term
- 3) Two bumper cars of masses 600 kg and 900 kg travelling (on a smooth surface) with velocities 8m/s and 4 m/s respectively, have a head on collision. If the coefficient of restitution is 0.5. a) What sort of collision is this? b) Calculate their velocities immediately after collision. c) If the coefficient of restitution was 1 instead of 0.5, what is the amount of energy lost during collision?arrow_forwardThe rectangular loop of wire shown in the figure (Figure 1) has a mass of 0.18 g per centimeter of length and is pivoted about side ab on a frictionless axis. The current in the wire is 8.5 A in the direction shown. Find the magnitude of the magnetic field parallel to the y-axis that will cause the loop to swing up until its plane makes an angle of 30.0 ∘ with the yz-plane. Find the direction of the magnetic field parallel to the y-axis that will cause the loop to swing up until its plane makes an angle of 30.0 ∘ with the yz-plane.arrow_forwardGive a more general expression for the magnitude of the torque τ. Rewrite the answer found in Part A in terms of the magnitude of the magnetic dipole moment of the current loop m. Define the angle between the vector perpendicular to the plane of the coil and the magnetic field to be ϕ, noting that this angle is the complement of angle θ in Part A. Give your answer in terms of the magnetic moment mm, magnetic field B, and ϕ.arrow_forward
- Calculate the electric and magnetic energy densities at thesurface of a 3-mm diameter copper wire carrying a 15-A current. The resistivity ofcopper is 1.68×10-8 Ω.m.Prob. 18, page 806, Ans: uE= 5.6 10-15 J/m3 uB= 1.6 J/m3arrow_forwardA 15.8-mW laser puts out a narrow beam 2.0 mm in diameter.Suppose that the beam is in free space. What is the rms value of E in the beam? What isthe rms value of B in the beam?Prob. 28, page 834. Ans: Erms= 1380 V/m, Brms =4.59×10-6 Tarrow_forwardA 4.5 cm tall object is placed 26 cm in front of a sphericalmirror. It is desired to produce a virtual image that is upright and 3.5 cm tall.(a) What type of mirror should be used, convex, or concave?(b) Where is the image located?(c) What is the focal length of the mirror?(d) What is the radius of curvature of the mirror?Prob. 25, page 861. Ans: (a) convex, (b) di= -20.2 cm, i.e. 20.2 cm behind the mirror,(c) f= -90.55 cm, (d) r= -181.1 cm.arrow_forward
- A series RCL circuit contains an inductor with inductance L=3.32 mH, and a generator whose rms voltage is 11.2 V. At a resonant frequencyof 1.25 kHz the average power delivered to the circuit is 26.9 W.(a) Find the value of the capacitance.(b) Find the value of the resistance.(c) What is the power factor of this circuit?Ans: C=4.89 μF, R=4.66 Ω, 1.arrow_forwardA group of particles is traveling in a magnetic field of unknown magnitude and direction. You observe that a proton moving at 1.70 km/s in the +x-direction experiences a force of 2.06×10−16 N in the +y-direction, and an electron moving at 4.40 km/s in the −z-direction experiences a force of 8.10×10−16 N in the +y-direction. What is the magnitude of the magnetic force on an electron moving in the −y-direction at 3.70 km/s ? What is the direction of this the magnetic force? (in the xz-plane)arrow_forwardA particle with a charge of −5.20 nC is moving in a uniform magnetic field of B =−( 1.22 T )k^. The magnetic force on the particle is measured to be F=−( 3.50×10−7 N )i^+( 7.60×10−7 N )j^. Calculate the x component of the velocity of the particle.arrow_forward
- Is it possible for average velocity to be negative?a. Yes, in cases when the net displacement is negative.b. Yes, if the body keeps changing its direction during motion.c. No, average velocity describes only magnitude and not the direction of motion.d. No, average velocity describes only the magnitude in the positive direction of motion.arrow_forwardTutorial Exercise An air-filled spherical capacitor is constructed with an inner-shell radius of 6.95 cm and an outer-shell radius of 14.5 cm. (a) Calculate the capacitance of the device. (b) What potential difference between the spheres results in a 4.00-μC charge on the capacitor? Part 1 of 4 - Conceptualize Since the separation between the inner and outer shells is much larger than a typical electronic capacitor with separation on the order of 0.1 mm and capacitance in the microfarad range, we expect the capacitance of this spherical configuration to be on the order of picofarads. The potential difference should be sufficiently low to avoid sparking through the air that separates the shells. Part 2 of 4 - Categorize We will calculate the capacitance from the equation for a spherical shell capacitor. We will then calculate the voltage found from Q = CAV.arrow_forwardI need help figuring out how to do part 2 with the information given in part 1 and putting it in to the simulation. ( trying to match the velocity graph from the paper onto the simulation to find the applied force graph) Using this simulation https://phet.colorado.edu/sims/cheerpj/forces-1d/latest/forces-1d.html?simulation=forces-1d.arrow_forward
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
 Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





