
Concept explainers
A vessel contains 1.00 × 104 oxygen molecules at 500 K. (a) Make an accurate graph of the Maxwell speed distribution function versus speed with points at speed intervals of 100 m/s. (b) Determine the most probable speed from this graph. (c) Calculate the average and rms speeds for the molecules and label these points on your graph. (d) From the graph, estimate the fraction of molecules with speeds in the range 300 m/s to 600 m/s.
(a)
The graph of the Maxwell speed distribution function versus speed with points at speed intervals of
Answer to Problem 38AP
The of the Maxwell speed distribution function versus speed with points at speed intervals of
Explanation of Solution
The Maxwell distribution curve is the graph between the distribution of speed and the change in speed or speed interval.
The number of molecules of oxygen in vessel is
Write the expression of Maxwell’s speed distribution function.
Here,
The mass of the molecules of oxygen
Here,
The molecular mass of the oxygen molecules in
Substitute
Substitute
Substitute the values of
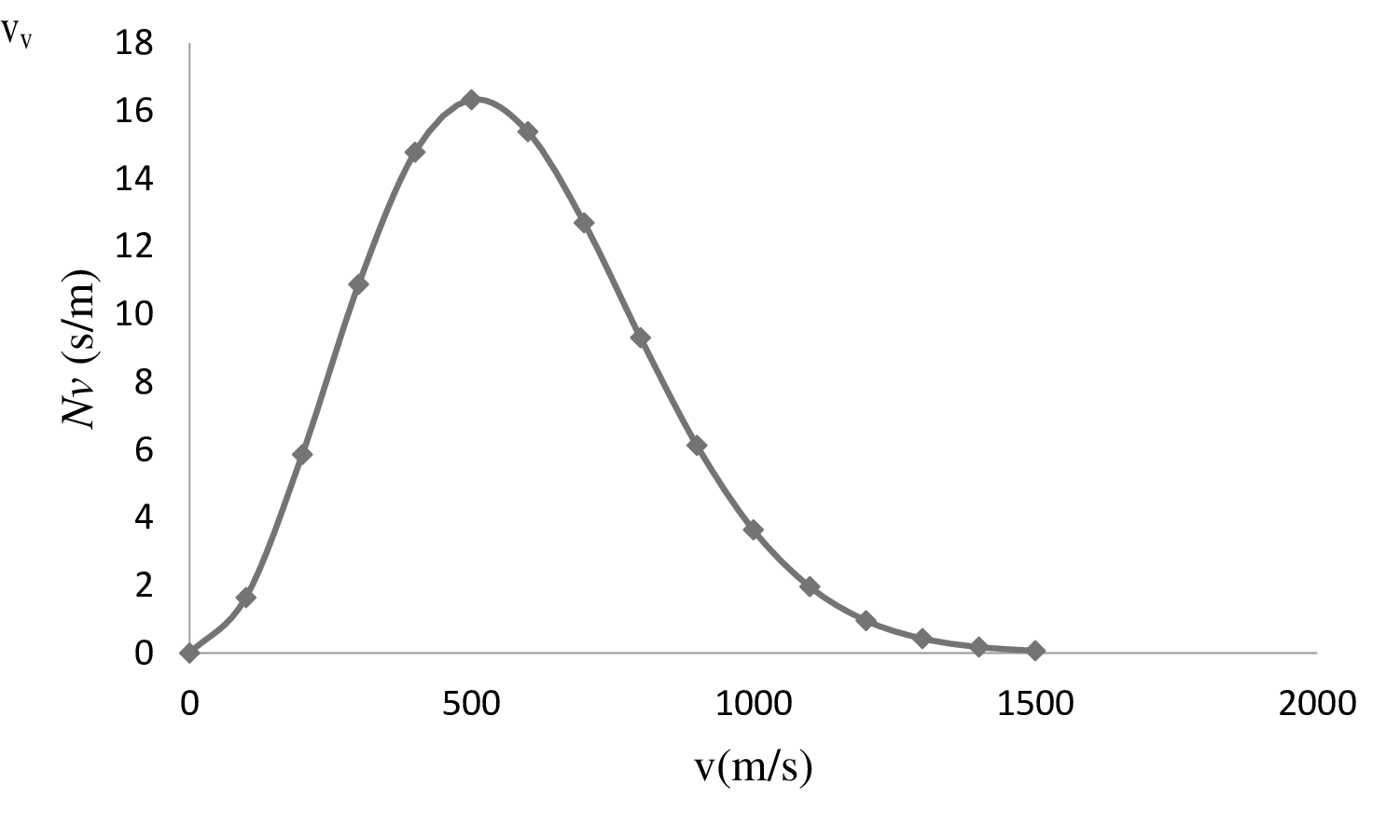
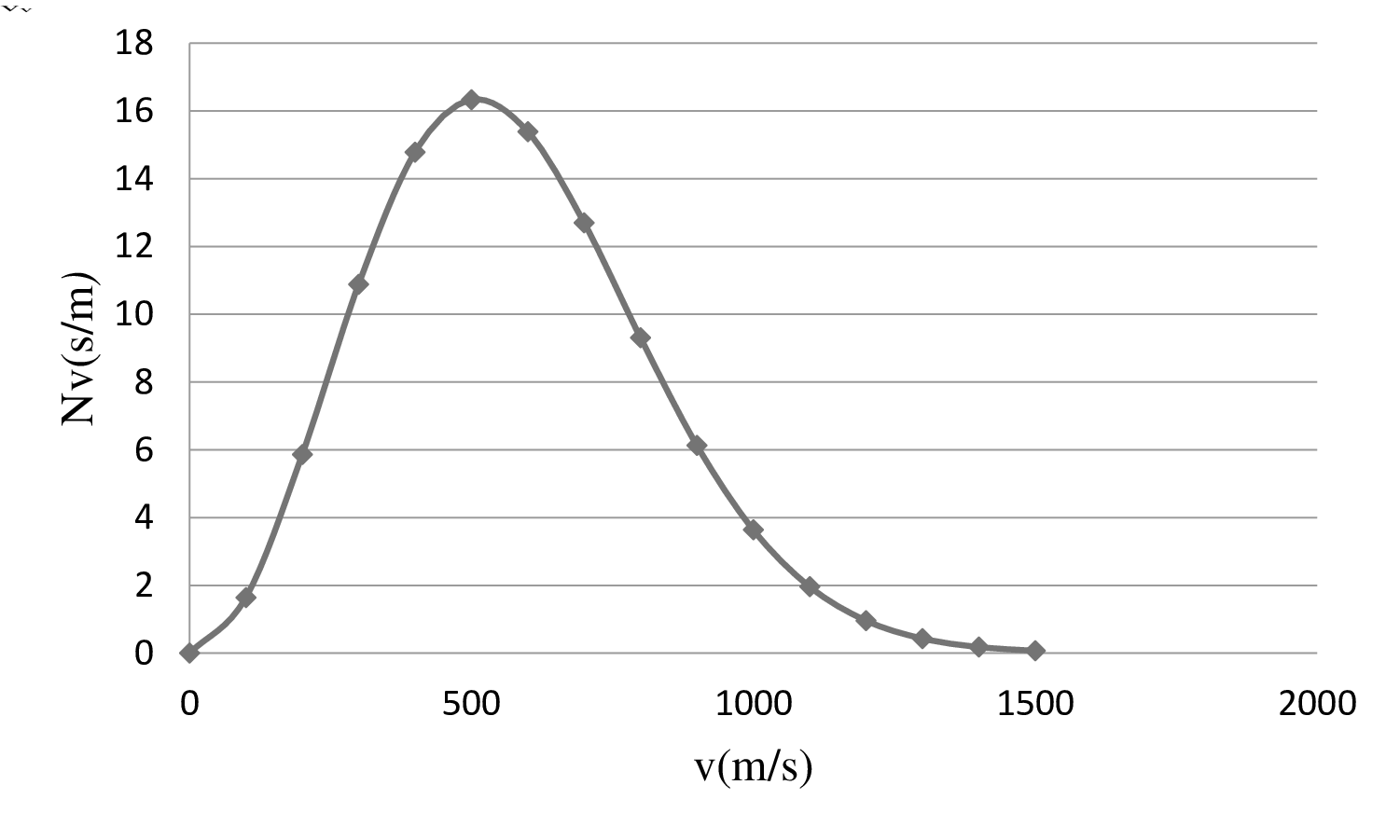
| 0 | 0 |
| 100 | 1.64 |
| 200 | 5.86 |
| 300 | 10.88 |
| 400 | 14.78 |
| 500 | 16.33 |
| 600 | 15.39 |
| 700 | 12.7 |
| 800 | 9.31 |
| 900 | 6.13 |
| 1000 | 3.64 |
| 1100 | 1.961 |
| 1200 | 0.96 |
| 1300 | 0.43 |
| 1400 | 0.18 |
| 1500 | 0.07 |
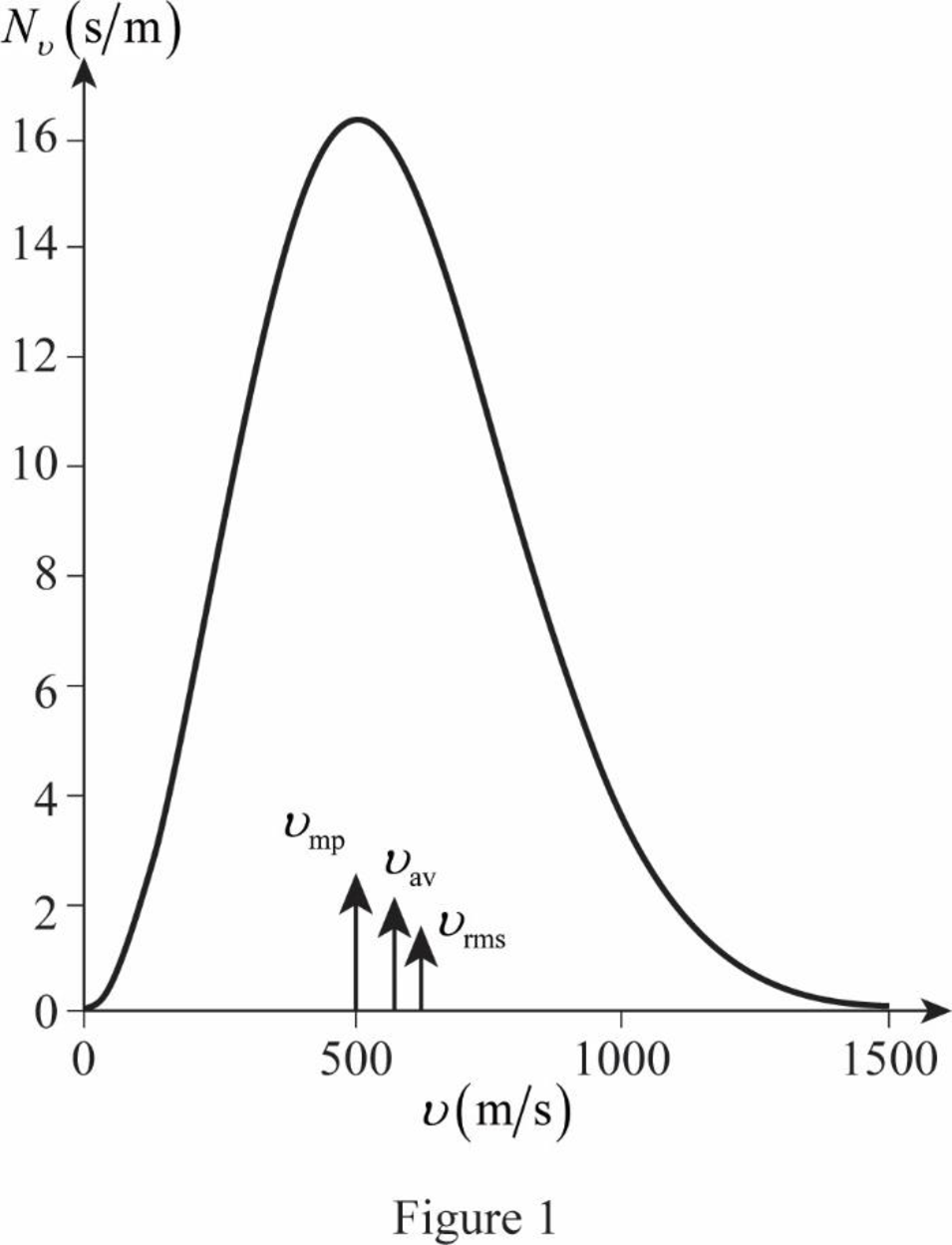
On the basis of the table, a graph is plotted below;
(b)
The most probable speed from the graph.
Answer to Problem 38AP
The most probable speed is
Explanation of Solution
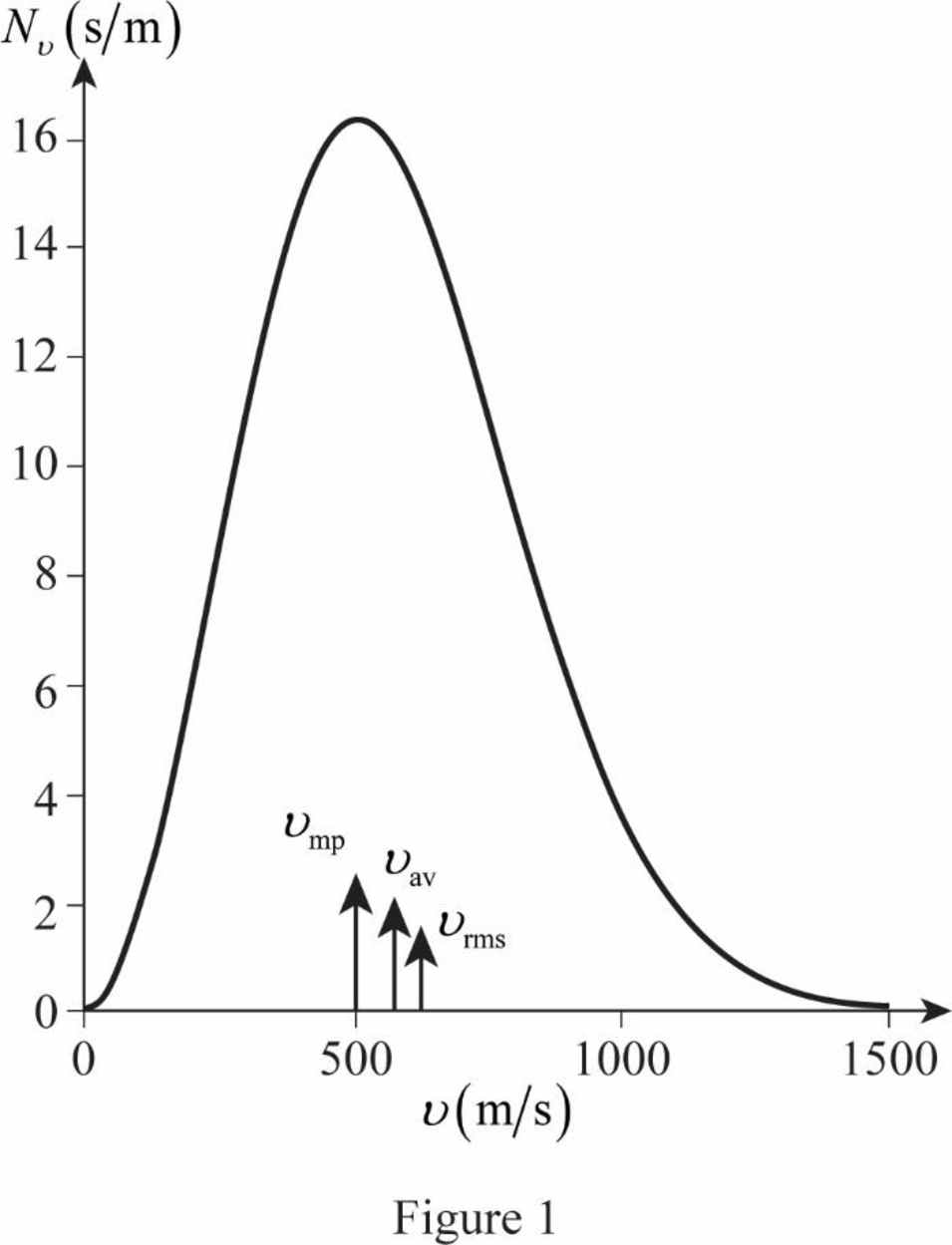
The most probable speed occurs where
Conclusion:
Therefore, the most probable speed is
(c)
The average and rms speeds for the molecules and label these points on the graph.
Answer to Problem 38AP
The average and rms speeds for the molecules is
Explanation of Solution
Write the expression of average velocity.
The mass of the molecules of oxygen
Substitute
The molecular mass of the oxygen molecules in
Substitute
Thus, the average speed is
Write the expression of rms velocity.
Substitute
Substitute
Thus, the rms velocity of the oxygen molecules is
The graph of Maxwell’s curve is shown below;
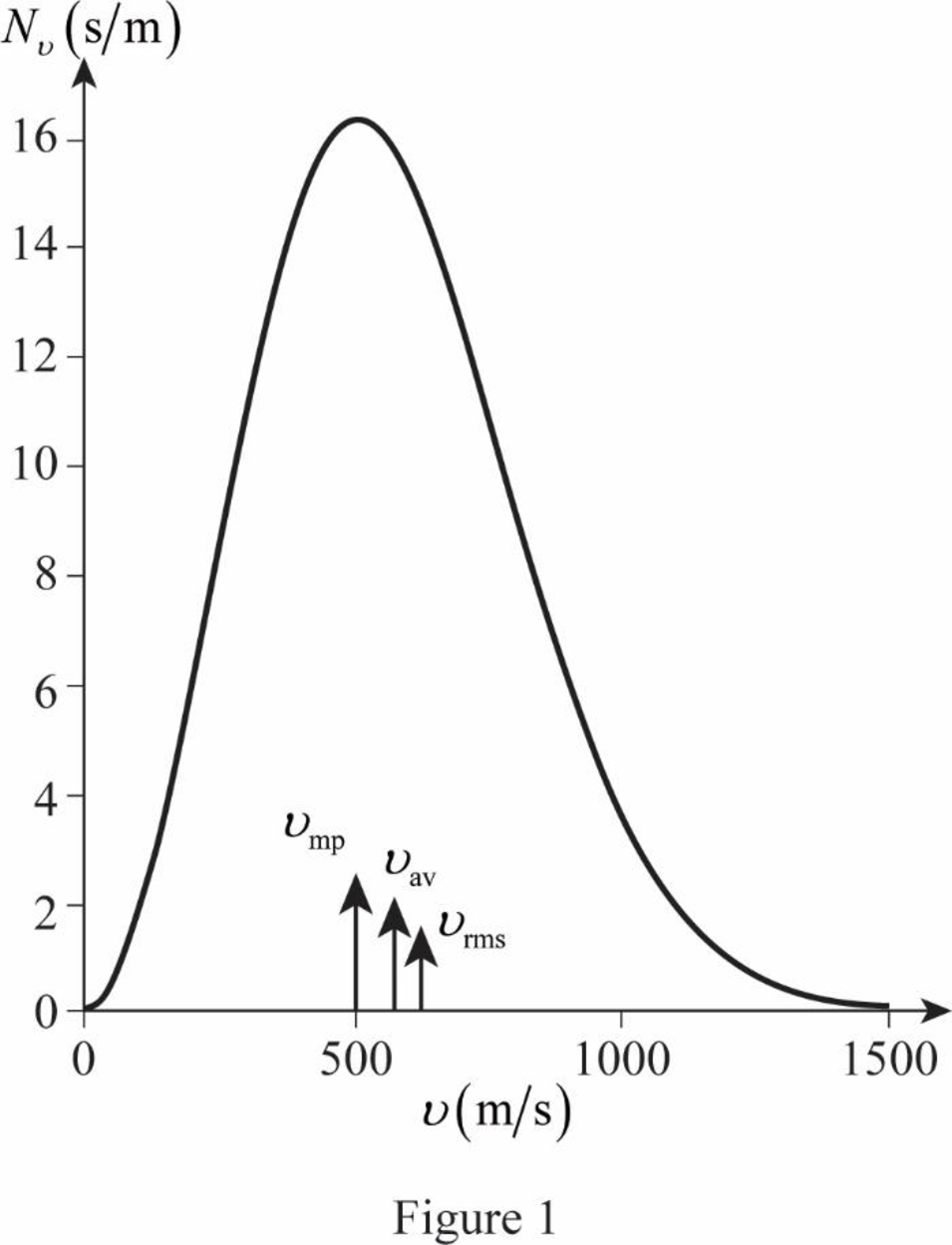
The point
Conclusion:
Therefore, the average and rms speeds for the molecules is
(d)
The fraction of molecules with the speed in the range of
Answer to Problem 38AP
The fraction of molecules with the speed in the range of
Explanation of Solution
The figure given below shows the Maxwell’s curve,
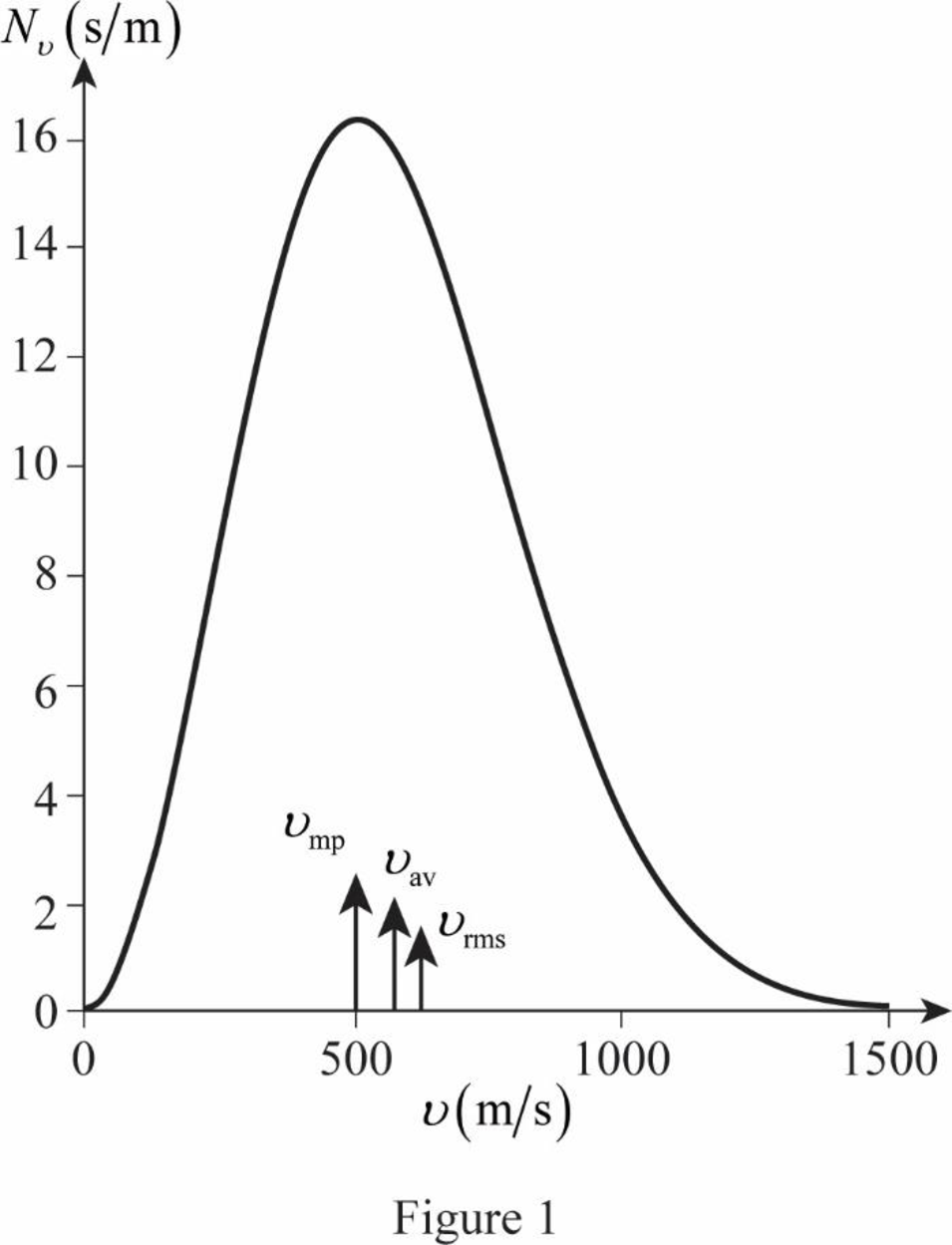
The area under the distribution curve in the range
Conclusion:
Write the area under the curve in the range
Therefore, the fraction of molecules with the speed in the range of
Want to see more full solutions like this?
Chapter 20 Solutions
Physics for Scientists and Engineers
- Question 6: Chlorine is widely used to purify municipal water supplies and to treat swimming pool waters. Suppose that the volume of a particular sample of Cl₂ gas is 8.70 L at 895 torr and 24°C. (a) How many grams of Cl₂ are in the sample? ⚫ Atomic mass of CI = 35.453 g/mol • Molar mass of Cl₂ = 2 x 35.453 = 70.906 g/mol Solution: Use the Ideal Gas Law: Step 1: Convert Given Values • Pressure: P = 895 torr → atm PV= = nRT 1 P = 895 × = 1.1789 atm 760 • Temperature: Convert to Kelvin: T24273.15 = 297.15 K • Gas constant: R = 0.0821 L atm/mol. K Volume: V = 8.70 L Step 2: Solve for n . PV n = RT n = (1.1789)(8.70) (0.0821)(297.15) 10.25 n = = 0.420 mol 24.405 Step 3: Calculate Mass of Cl₂ Final Answer: 29.78 g of Cl₂. mass nx M mass= (0.420)(70.906) mass= 29.78 garrow_forwardE1 R₁ w 0.50 20 Ω 12 R₁₂ ww ΒΩ R₂ 60 E3 C RA w 15 Ω E2 0.25 E4 0.75 Ω 0.5 Ωarrow_forwardSolve plzarrow_forward
- how would i express force in vector form I keep getting a single numberarrow_forwardplease help me solve this questions. show all calculations and a good graph too :)arrow_forwardWhat is the force (in N) on the 2.0 μC charge placed at the center of the square shown below? (Express your answer in vector form.) 5.0 με 4.0 με 2.0 με + 1.0 m 1.0 m -40 με 2.0 μCarrow_forward
- What is the force (in N) on the 5.4 µC charge shown below? (Express your answer in vector form.) −3.1 µC5.4 µC9.2 µC6.4 µCarrow_forwardAn ideal gas in a sealed container starts out at a pressure of 8900 N/m2 and a volume of 5.7 m3. If the gas expands to a volume of 6.3 m3 while the pressure is held constant (still at 8900 N/m2), how much work is done by the gas? Give your answer as the number of Joules.arrow_forwardThe outside temperature is 25 °C. A heat engine operates in the environment (Tc = 25 °C) at 50% efficiency. How hot does it need to get the high temperature up to in Celsius?arrow_forward
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
 Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





