
FUND.OF PHYSICS(LL)-PRINT COMP-W/ACCESS
11th Edition
ISBN: 9781119455608
Author: Halliday
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 20, Problem 33P
SSM ILW Figure 20-29 shows a reversible cycle through which 1.00 mol of a monatomic ideal gas is taken. Volume Vc = 8.00 Vb. Process bc is an adiabatic expansion, with pb = 10.0 atm and Vb = 1.00 × 10−3 m3. For the cycle, find (a) the energy added to the gas as heat, (b) the energy leaving the gas as heat, (c) the net work done by the gas, and (d) the efficiency of the cycle.
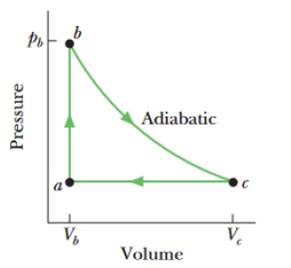
Figure 20-29 Problem 33.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
3.63 • Leaping the River II. A physics professor did daredevil
stunts in his spare time. His last stunt was an attempt to jump across
a river on a motorcycle (Fig. P3.63). The takeoff ramp was inclined at
53.0°, the river was 40.0 m wide, and the far bank was 15.0 m lower
than the top of the ramp. The river itself was 100 m below the ramp.
Ignore air resistance. (a) What should his speed have been at the top of
the ramp to have just made it to the edge of the far bank? (b) If his speed
was only half the value found in part (a), where did he land?
Figure P3.63
53.0°
100 m
40.0 m→
15.0 m
Please solve and answer the question correctly please. Thank you!!
You throw a small rock straight up from the edge of a highway bridge that crosses a river. The rock passes you on its way down, 5.00 s after it was thrown. What is the speed of the rock just before it reaches the water 25.0 m below the point where the rock left your hand? Ignore air resistance.
Chapter 20 Solutions
FUND.OF PHYSICS(LL)-PRINT COMP-W/ACCESS
Ch. 20 - Point i in Fig. 20-19 represents the initial state...Ch. 20 - In lour experiments, blocks A and B, starting ill...Ch. 20 - A gas, confined to an insulated cylinder, is...Ch. 20 - An ideal monatomic gas at initial temperature T0...Ch. 20 - In four experiments, 2.5 mol of hydrogen gas...Ch. 20 - A box contains 100 atoms in a configuration that...Ch. 20 - Does the entropy per cycle increase, decrease, or...Ch. 20 - Three Carnot engines operate between temperature...Ch. 20 - An inventor claims to have invented four engines,...Ch. 20 - Does the entropy per cycle increase, decrease, or...
Ch. 20 - SSM Suppose 4.00 mol of an ideal gas undergoes a...Ch. 20 - Prob. 2PCh. 20 - ILW A 2.50 mol sample of an ideal gas expands...Ch. 20 - Prob. 4PCh. 20 - ILW Find a the energy absorbed as heat and b the...Ch. 20 - a What is the entropy change of a 12.0 g ice cube...Ch. 20 - ILW A 50.0 g block of copper whose temperature is...Ch. 20 - At very low temperatures, the molar specific heat...Ch. 20 - A 10 g ice cube at 10oC is placed in a lake whose...Ch. 20 - A 364 g block is put in contact with a thermal...Ch. 20 - SSM WWW In an experiment, 200 g of aluminum with a...Ch. 20 - A gas sample undergoes a reversible isothermal...Ch. 20 - In the irreversible process of Fig. 20-5, let the...Ch. 20 - Prob. 14PCh. 20 - A mixture of 1773 g of water and 227 g of ice is...Ch. 20 - GO An 8.0 g ice cube at -10C is put into a Thermos...Ch. 20 - Prob. 17PCh. 20 - GO A 2.0 mol sample of an ideal monatomic gas...Ch. 20 - Suppose 1.00 mol of a monatomic ideal gas is taken...Ch. 20 - Expand 1.00 mol of an monatomic gas initially at...Ch. 20 - GO Energy can be removed from water as heat at and...Ch. 20 - GO An insulated Thermos contains 130 g of water at...Ch. 20 - A Carnot engine whose low-temperature reservoir is...Ch. 20 - A Carnot engine absorbs 52 kJ as heat and exhausts...Ch. 20 - A Carnot engine has an efficiency of 22.0. It...Ch. 20 - In a hypothetical nuclear fusion reactor, the fuel...Ch. 20 - SSM WWW A Carnot engine operates between 235C and...Ch. 20 - In the first stage of a two-stage Carnot engine,...Ch. 20 - GO Figure 20-27 shows a reversible cycle through...Ch. 20 - A 500 W Carnot engine operates between...Ch. 20 - The efficiency of a particular car engine is 25...Ch. 20 - GO A Carnot engine is set up to produce a certain...Ch. 20 - SSM ILW Figure 20-29 shows a reversible cycle...Ch. 20 - GO An ideal gas 1.0 mol is the working substance...Ch. 20 - The cycle in Fig. 20-31 represents the operation...Ch. 20 - How much work must be done by a Carnot...Ch. 20 - SSM A heat pump is used to heal a building, The...Ch. 20 - The electric motor of a heat pump transfers energy...Ch. 20 - SSM A Carnot air conditioner lakes energy from the...Ch. 20 - To make ice, a freezer that is a reverse Carnot...Ch. 20 - ILW An air conditioner operating between 93F and...Ch. 20 - The motor in a refrigerator has a power of 200 W....Ch. 20 - GO Figure 20-32 represents a Carnot engine that...Ch. 20 - a During each cycle, a Carnot engine absorbs 750 J...Ch. 20 - Prob. 45PCh. 20 - A box contains N identical gas molecules equally...Ch. 20 - SSM WWW A box contains N gas molecules, Consider...Ch. 20 - Four particles are in the insulated box of Fig....Ch. 20 - A cylindrical copper rod of length 1.50 m and...Ch. 20 - Suppose 0.550 mol of an ideal gas is isothermally...Ch. 20 - Prob. 51PCh. 20 - Suppose 1.0 mol of a monatomic ideal gas initially...Ch. 20 - GO Suppose that a deep shaft were drilled in...Ch. 20 - What is the entropy change for 3.20 mol of an...Ch. 20 - A 600 g lump of copper at 80.0C is placed in 70.0...Ch. 20 - Figure 20-33 gives the force magnitude F versus...Ch. 20 - The temperature of 1.00 mol of a monatomic ideal...Ch. 20 - Repeat Problem 57, with the pressure now kept...Ch. 20 - Prob. 59PCh. 20 - A three-step cycle is undergone by 3.4 mol of an...Ch. 20 - An inventor has built an engine X and claims that...Ch. 20 - Suppose 2.00 mol of a diatomic gas is taken...Ch. 20 - A three-step cycle is undergone reversibly by 4.00...Ch. 20 - a A Carnot engine operates between a hot reservoir...Ch. 20 - A 2.00 mol diatomic gas initially at 300 K...Ch. 20 - An ideal refrigerator does 150 J of work to remove...Ch. 20 - Suppose that 260 J is conducted from a...Ch. 20 - An apparatus that liquefies helium is in a room...Ch. 20 - GO A brass rod is in thermal contact with a...Ch. 20 - A 45.0 g block of tungsten at 30.0C and a 25.0 g...Ch. 20 - Prob. 71PCh. 20 - Calculate the efficiency of a fossil-fuel power...Ch. 20 - SSM A Carnot refrigerator extracts 35.0 kJ as heat...Ch. 20 - A Carnot engine whose high-temperature reservoir...Ch. 20 - SSM System A of three particles and system B of...Ch. 20 - Figure 20-36 shows a Carnot cycle on a T-S...Ch. 20 - Find the relation between the efficiency of a...Ch. 20 - A Carnot engine has a power of 500 W. It operates...Ch. 20 - In a real refrigerator, the low-temperature coils...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Define histology.
Fundamentals of Anatomy & Physiology (11th Edition)
How Would the experiments result charge if oxygen (O2) were induced in the spark chamber?
Biology: Life on Earth with Physiology (11th Edition)
7. Which bones form via intramembranous ossification?
a. Irregular bones
b. Certain flat bones
c. Long bones
d....
Human Anatomy & Physiology (2nd Edition)
17.33 On heating, cis-4-hydroxycyclohexanecarboxylic acid forms a lactone but trans-4-hydroxycyclohexanecarboxy...
Organic Chemistry
Two culture media were inoculated with four different bacteria. After incubation, the following results were ob...
Microbiology: An Introduction
Choose the best answer to each of the following. Explain your reasoning. When we observe a distant galaxy whose...
Cosmic Perspective Fundamentals
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Help me make a visualize experimental setup using a word document. For the theory below.arrow_forwardHow to solve this, given answerarrow_forwardThree point-like charges are placed at the corners of a square as shown in the figure, 28.0 cm on each side. Find the minimum amount of work required by an external force to move the charge q1 to infinity. Let q1=-2.10 μC, q2=+2.40 μС, q3=+3.60 μC.arrow_forward
- A point charge of -4.00 nC is at the origin, and a second point charge of 6.00 nC is on the x axis at x= 0.820 mm . Find the magnitude and direction of the electric field at each of the following points on the x axis. x2 = 19.0 cmarrow_forwardFour point-like charges are placed as shown in the figure, three of them are at the corners and one at the center of a square, 36.0 cm on each side. What is the electric potential at the empty corner? Let q1=q3=+26.0 µС, q2=-28.0 μC, and q4=-48.0μc Varrow_forwardPLS HELparrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning


Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
The Second Law of Thermodynamics: Heat Flow, Entropy, and Microstates; Author: Professor Dave Explains;https://www.youtube.com/watch?v=MrwW4w2nAMc;License: Standard YouTube License, CC-BY