
(a)
Interpretation:
The approximate concentration of A remaining after 110 sec for zero order reaction is to be determined.
| Set I | Set II | Set III | |||
| Time, s | [A], M | Time, s | [A], M | Time, s | [A], M |
| 0 | 1.00 | 0 | 1.00 | 0 | 1.00 |
| 25 | 0.78 | 25 | 0.75 | 25 | 0.80 |
| 50 | 0.61 | 50 | 0.50 | 50 | 0.67 |
| 75 | 0.47 | 75 | 0.25 | 75 | 0.57 |
| 100 | 0.37 | 100 | 0.00 | 100 | 0.50 |
| 150 | 0.22 | 150 | 0.40 | ||
| 200 | 0.14 | 200 | 0.33 | ||
| 250 | 0.08 | 250 | 0.29 |
Concept introduction:
- Zero-order reaction- for this,
rate of reaction is independent of the concentration.
And the graph of zero order reaction is-

Integral rate law for zero order reaction is −
(b)
Interpretation:
The approximate concentration of A remaining after 110 sec for first order reaction is to be determined.
| Set I | Set II | Set III | |||
| Time, s | [A], M | Time, s | [A], M | Time, s | [A], M |
| 0 | 1.00 | 0 | 1.00 | 0 | 1.00 |
| 25 | 0.78 | 25 | 0.75 | 25 | 0.80 |
| 50 | 0.61 | 50 | 0.50 | 50 | 0.67 |
| 75 | 0.47 | 75 | 0.25 | 75 | 0.57 |
| 100 | 0.37 | 100 | 0.00 | 100 | 0.50 |
| 150 | 0.22 | 150 | 0.40 | ||
| 200 | 0.14 | 200 | 0.33 | ||
| 250 | 0.08 | 250 | 0.29 |
Concept introduction:
- The first order reaction is the one in which the rate is directly proportional to the concentration of one of the reactant.
And half-life is
Integral rate law for first order reaction is −
And
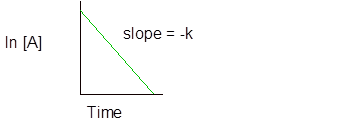
(c)
Interpretation:
The approximate concentration of A remaining after 110 sec for second order reaction is to be determined.
| Set I | Set II | Set III | |||
| Time, s | [A], M | Time, s | [A], M | Time, s | [A], M |
| 0 | 1.00 | 0 | 1.00 | 0 | 1.00 |
| 25 | 0.78 | 25 | 0.75 | 25 | 0.80 |
| 50 | 0.61 | 50 | 0.50 | 50 | 0.67 |
| 75 | 0.47 | 75 | 0.25 | 75 | 0.57 |
| 100 | 0.37 | 100 | 0.00 | 100 | 0.50 |
| 150 | 0.22 | 150 | 0.40 | ||
| 200 | 0.14 | 200 | 0.33 | ||
| 250 | 0.08 | 250 | 0.29 |
Concept introduction:
- The second order reaction is the one in which the rate is directly proportional to the square of the concentration of one of the reactant.
Integral rate law for second order reaction is −
And the graph of second order reaction is-
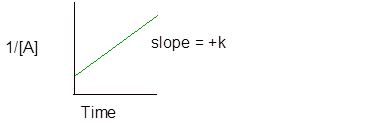
Want to see the full answer?
Check out a sample textbook solution
Chapter 20 Solutions
General Chemistry: Principles And Modern Applications Plus Mastering Chemistry With Pearson Etext -- Access Card Package (11th Edition)
- 4. The superoxide ion, Oz, plays an important role in the ageing processes that take place in organisms. Judge whether Oz is likely to have larger or smaller dissociation energy than 02. Molecular Orbital Diagram 02 02 Does O2 have larger or smaller dissociation energy?: Bond Orderarrow_forward1. How many molecular orbitals can be built from the valence shell orbitals in O2?arrow_forwardSho reaction mechanism. Don't give Ai generated solutionarrow_forward
- Is this aromatic, antiaromatic, or nonaromatic?arrow_forwardOn what basis are Na and Nb ranked against each other?arrow_forwardStep 1: add a curved arrow. Select Draw Templates More / " C H Br 0 Br : :o: Erase H H H H Q2Q Step 2: Draw the intermediates and a curved arrow. Select Draw Templates More MacBook Air / " C H Br 0 9 Q Erase 2Qarrow_forward
- O Macmillan Learning Question 23 of 26 > Stacked Step 7: Check your work. Does your synthesis strategy give a substitution reaction with the expected regiochemistry and stereochemistry? Draw the expected product of the forward reaction. - - CN DMF MacBook Air Clearly show stereochemistry. Questionarrow_forwardNH2 1. CH3–MgCl 2. H3O+ ? As the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will make a molecule with a new C - C bond as its major product: If this reaction will work, draw the major organic product or products you would expect in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If the major products of this reaction won't have a new C - C bond, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. This reaction will not make a product with a new C - C bond. Х ☐: Carrow_forwardPredict the major products of this organic reaction. If there will be no major products, check the box under the drawing area instead. No reaction. : + Х è OH K Cr O 2 27 2 4' 2 Click and drag to start drawing a structure.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





