
EBK CONCEPTS OF GENETICS
12th Edition
ISBN: 9780134818979
Author: Killian
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 20, Problem 31ESP
Most of the techniques described in this chapter (blotting, cloning, PCR, etc.) are dependent on hybridization (annealing) between different populations of
Tm = 81.5 + 16.6(logM[Na+]) + 0.41(%GC) − 0.72(%formamide)
- (a) For the following concentrations of Na+ and formamide, calculate the Tm. Assume 45% GC content.
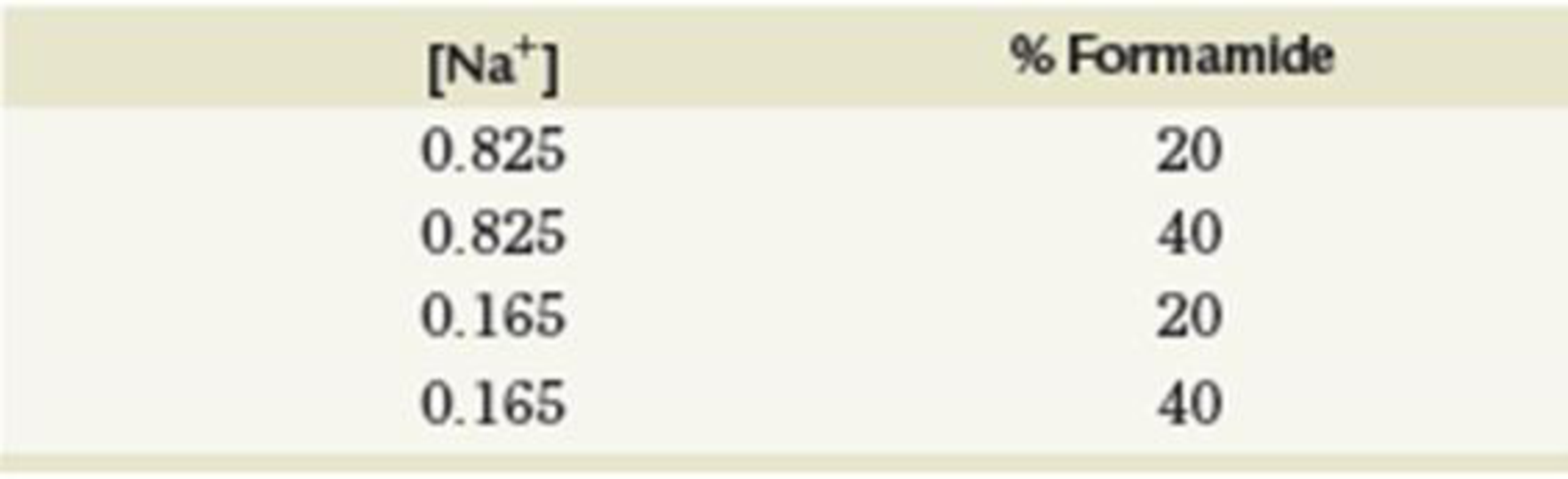
- (b) Given that formamide competes for hydrogen bond locations on nucleic acid bases and monovalent cations are attracted to the negative charges on nucleic acids, explain why the Tm varies as described in part (a).
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
10. Your instructor will give you 2 amino acids during the activity session (video 2-7.
A. First color all the polar and non-polar covalent bonds in the R groups of your 2 amino acids
using the same colors as in #7. Do not color the bonds in the backbone of each amino acid.
B. Next, color where all the hydrogen bonds, hydrophobic interactions and ionic bonds could
occur in the R group of each amino acid. Use the same colors as in #7. Do not color the bonds
in the backbone of each amino acid.
C. Position the two amino acids on the page below in an orientation where the two R groups
could bond together. Once you are satisfied, staple or tape the amino acids in place and label
the bond that you formed between the two R groups.
- Polar covalent Bond - Red
- Non polar Covalent boND- yellow
- Ionic BonD - PINK
Hydrogen Bonn - Purple
Hydrophobic interaction-green
O=C-N
H
I.
H
HO
H
=O
CH2
C-C-N
HICK
H
HO
H
CH2
OH
H₂N
C = O
Find the dental formula and enter it in the following format:
I3/3 C1/1 P4/4 M2/3 = 42 (this is not the correct number, just the correct format)
Please be aware: the upper jaw is intact (all teeth are present). The bottom jaw/mandible is not intact. The front teeth should include 6 total rectangular teeth (3 on each side) and 2 total large triangular teeth (1 on each side).
12. Calculate the area of a circle which has a radius of 1200 μm. Give your answer in mm² in
scientific notation with the correct number of significant figures.
Chapter 20 Solutions
EBK CONCEPTS OF GENETICS
Ch. 20 - A plasmid that is both ampicillin and tetracycline...Ch. 20 - You have just created the worlds first genomic...Ch. 20 - What undesirable or unforeseen consequences might...Ch. 20 - Do we have the ethical right to alter the genomes...Ch. 20 - Should these new technologies be regulated...Ch. 20 - HOW DO WE KNOW? In this chapter we focused on how...Ch. 20 - CONCEPT QUESTION Review the Chapter Concepts list...Ch. 20 - What roles do restriction enzymes, vectors, and...Ch. 20 - The human insulin gene contains a number of...Ch. 20 - Although many cloning applications involve...
Ch. 20 - Using DNA sequencing on a cloned DNA segment, you...Ch. 20 - Restriction sites are palindromic; that is, they...Ch. 20 - List the advantages and disadvantages of using...Ch. 20 - What are the advantages of using a restriction...Ch. 20 - In 1975, the Asilomar Conference on Recombinant...Ch. 20 - In the context of recombinant DNA technology, of...Ch. 20 - If you performed a PCR experiment starting with...Ch. 20 - Prob. 13PDQCh. 20 - Prob. 14PDQCh. 20 - You have recovered a cloned DNA segment from a...Ch. 20 - Prob. 16PDQCh. 20 - Although the capture and trading of great apes has...Ch. 20 - Prob. 18PDQCh. 20 - Prob. 19PDQCh. 20 - Prob. 20PDQCh. 20 - Traditional Sanger sequencing has largely been...Ch. 20 - How is fluorescent in situ hybridization (FISH)...Ch. 20 - What is the difference between a knockout animal...Ch. 20 - Prob. 24PDQCh. 20 - When disrupting a mouse gene by knockout, why is...Ch. 20 - Prob. 26PDQCh. 20 - Prob. 27PDQCh. 20 - As you will learn later in the text (Special...Ch. 20 - The gel presented here shows the pattern of bands...Ch. 20 - A widely used method for calculating the annealing...Ch. 20 - Most of the techniques described in this chapter...Ch. 20 - In humans, congenital heart disease is a common...Ch. 20 - The U.S. Department of Justice has established a...Ch. 20 - Prob. 34ESP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- Describe the image quality of the B.megaterium at 1000X before adding oil? What does adding oil do to the quality of the image?arrow_forwardWhich of the follwowing cells from this lab do you expect to have a nucleus and why or why not? Ceratium, Bacillus megaterium and Cheek epithelial cells?arrow_forward14. If you determine there to be debris on your ocular lens, explain what is the best way to clean it off without damaging the lens?arrow_forward
- 11. Write a simple formula for converting mm to μm when the number of mm's is known. Use the variable X to represent the number of mm's in your formula.arrow_forward13. When a smear containing cells is dried, the cells shrink due to the loss of water. What technique could you use to visualize and measure living cells without heat-fixing them? Hint: you did this technique in part I.arrow_forward10. Write a simple formula for converting μm to mm when the number of μm's are known. Use the variable X to represent the number of um's in your formula.arrow_forward
- 8. How many μm² is in one cm²; express the result in scientific notation. Show your calculations. 1 cm = 10 mm; 1 mm = 1000 μmarrow_forwardFind the dental formula and enter it in the following format: I3/3 C1/1 P4/4 M2/3 = 42 (this is not the correct number, just the correct format) Please be aware: the upper jaw is intact (all teeth are present). The bottom jaw/mandible is not intact. The front teeth should include 6 total rectangular teeth (3 on each side) and 2 total large triangular teeth (1 on each side).arrow_forwardAnswer iarrow_forward
- Answerarrow_forwardcalculate the questions showing the solution including variables,unit and equations all the questiosn below using the data a) B1, b) B2, c) hybrid rate constant (1) d) hybrid rate constant (2) e) t1/2,dist f) t1/2,elim g) k10 h) k12 i) k21 j) initial concentration (C0) k) central compartment volume (V1) l) steady-state volume (Vss) m) clearance (CL) AUC (0→10 min) using trapezoidal rule n) AUC (20→30 min) using trapezoidal rule o) AUCtail (AUC360→∞) p) total AUC (using short cut method) q) volume from AUC (VAUC)arrow_forwardQUESTION 8 For the following pedigree, assume that the mode of inheritance is X-linked recessive, and that the trait has full penetrance and expressivity and occurs at a very low frequency in the hum population. Using XA for the dominant allele and Xa for the recessive allele, assign genotypes for the following individuals (if it is not possible to figure out the second allele of a genotype, that with an underscore): 2 m 1 2 1 2 4 5 6 7 8 9 IV 1 2 3 5 6 7 8 CO 9 10 12 13 V 1, 2 3 4 5 6 7 8 9 10 11 12 13 a. Il-1: b. 11-2: c. III-3: d. III-4: e. If individuals IV-11 and IV-12 have another child, what is the probability that they will have a boy with the disorder?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning


Human Heredity: Principles and Issues (MindTap Co...
Biology
ISBN:9781305251052
Author:Michael Cummings
Publisher:Cengage Learning

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:9781305389892
Author:Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:Cengage Learning

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax


Biology Today and Tomorrow without Physiology (Mi...
Biology
ISBN:9781305117396
Author:Cecie Starr, Christine Evers, Lisa Starr
Publisher:Cengage Learning
Molecular Techniques: Basic Concepts; Author: Dr. A's Clinical Lab Videos;https://www.youtube.com/watch?v=7HFHZy8h6z0;License: Standard Youtube License