
Concept explainers
Interpretation:
The structural formula for each of the given compounds is to be drawn.
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
Rules for writing the structural formula from IUPAC are:
First identify the word root for the given compound.
The suffix used in the compound like –ane, ene, yne, ol, al and so on.
Identify the position, location, and number of the substituent bonded to the carbon chain.
Answer to Problem 28P
Solution:
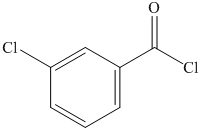
a) The structural formula of m-chlorobenzoyl chlorideis shown below.
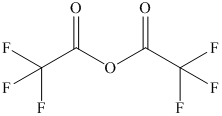
b) The structural formula of trifluoroacetic anhydride is shown below.
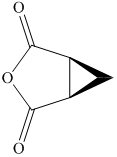
c) The structural formula of cis-
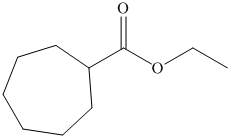
d) The structural formula of ethyl cycloheptanecarboxylateis shown below.
(e) The structural formula of
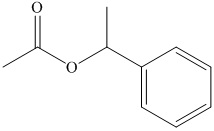
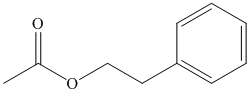
(f) The structural formula of
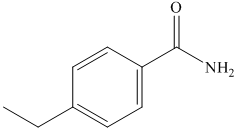
(g) The structural formula of p-ethylbenzamide is shown below.
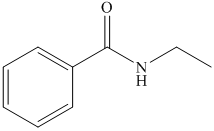
(h) The structural formula of N-ethylbenzamide is shown below.
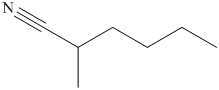
(i) The structural formula of
Explanation of Solution
a) m-Chlorobenzoyl chloride.
The name of compound suggests that chlorine group is present at meta position with respect to acyl group on benzene ring. The structure of compound is shown below.

b) Trifluoroacetic anhydride.
The name of the compound shows that anhydride group
group. The structure of compound is shown below.

c) cis-
The name of the compound indicates that two carboxylic groups are present on cyclopropane. Both carboxylic groups are present on the same plane. The structure of compound is shown below.

d) Ethyl cycloheptanecarboxylate
The name of the compound suggests that ester group is present in the structure. The cycloheptane ring is bonded to carbonyl carbon atom and ethyl group is bonded to oxygen atom. The structure of compound is shown below.

(e)
The name of the compound suggests that ester group is present in the structure. The methyl group is bonded to carbonyl carbon atom and ethyl group is bonded to oxygen atom. The structure of compound is shown below.

(f)
The name of the compound suggests that ester group is present in the structure. The methyl group is bonded to carbonyl carbon atom and ethyl group is bonded to oxygen atom. The second carbon atom is bonded to benzene ring. The structure of compound is shown below.

(g) p-Ethylbenzamide
The name of compound suggests that ethyl group is present at para position with respect to amide group on benzene ring. The structure of compound is shown below.

(h) N-Ethylbenzamide
The name of compound suggests that ethyl group is bonded to amide group which further bonded to benzene ring. The structure of compound is shown below.

(i)
The name of the compound indicates that it contains hydrocarbon chain of six carbon atoms. On the second carbon atom methyl group is present and on the first carbon atom nitrile group is present. The structure of compound is shown below.

Want to see more full solutions like this?
Chapter 20 Solutions
ORGANIC CHEMISTRY (LL)-W/SOLN.>CUSTOM<
- What is the product of the reaction shown below? Why is the correct answer D? Please explain what is happening. Please include a detailed explanation and a drawing of steps needed to understand the reaction or question.arrow_forwardWrite the systematic name of each organic molecule: structure name П O ☐ O ☐ Oarrow_forwardThe 13C NMR signal for which of the indicated carbons will occur at the frequency (most deshielded)? Why is the correct answer E? Please explain what is happening. Please include a detailed explanation needed to understand the or question.arrow_forward
- Which of the following reagents best achieves the reaction shown below? Why is the correct answer B? Please explain what is happening. Please include a detailed explanation and a drawing of steps needed to understand the reaction or question.arrow_forwardWhat is the product of the following reaction sequence? Why is the correct answer D? Please explain what is happening. Please include a detailed explanation and a drawing of steps needed to understand the reaction or question.arrow_forwardPls help ASAParrow_forward
- The reaction of phenylmagnesium bromide (C6H5MgBr) with propanal (CH3CH2CHO)3 followed by hydrolysis yields. A. 2-phenyl-1-propanol B. 1-phenyl-1propanol C. 3-phenyl-2-propanol D. 3-phenyl-1-propanol Why is the correct answer B? Please explain what is happening. Please include a detailed explanation and/or a drawing of steps needed to understand the reaction or question.arrow_forwardWhat is the product of the reaction sequence below? Why is the correct answer D? Please explain what is happening. Please include a detailed explanation and a drawing of steps needed to understand the reaction or question. The part that is under the pen in the image is (1) CH3CHO (2) H3O+arrow_forwardWhat is the missing reactant in this organic reaction? R+ OH HD CH3-CH2-CH-CH3 H A CH3 CH3-CH2-C-O-CH-CH2-CH3 + H₂O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. No answer Click anywhere to draw the first atom of your structure. ☐ : Jm +arrow_forward
- Draw the major product of the following E2 reaction. Make sure you pay attention to REGIOCHEMISTRY and STEREOCHEMISTRY. Explain why this product is formed using 10 words or less for each. (a) NaH Br acetone TSO, NaH (b) acetonearrow_forward2. Circle the compound that will react SLOWER in an E2 reaction. To get credit for this question, you must EXPLAIN how you got your answer using STRUCTURES and WORDS. Br ** Br...arrow_forward8. 2 20 00 Draw ALL of the possible products for the following reaction CIRCLE the MAJOR product NaOMe MeOHarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





