
Concept explainers
(a)
Interpretation: The mechanism for the formation of compound A has to be proposed.
Concept Introduction:
Diels-Alder reaction:
It is the reaction of conjugated dienes with double or triple bonded compounds which are known as “dienophiles”. The reaction is a
Example:
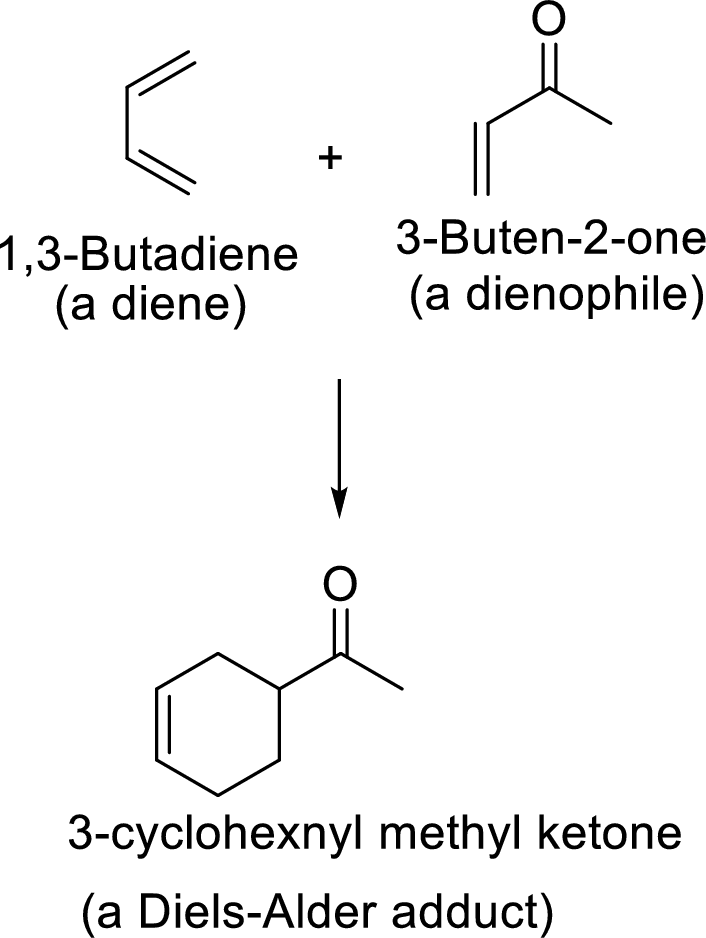
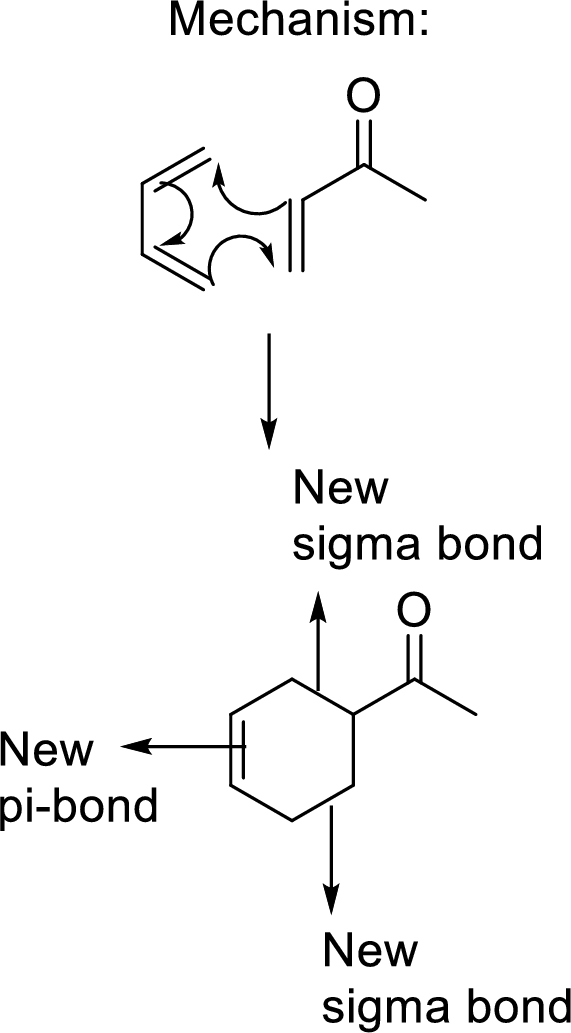
This mechanism shown that three
Formation of Benzyne:
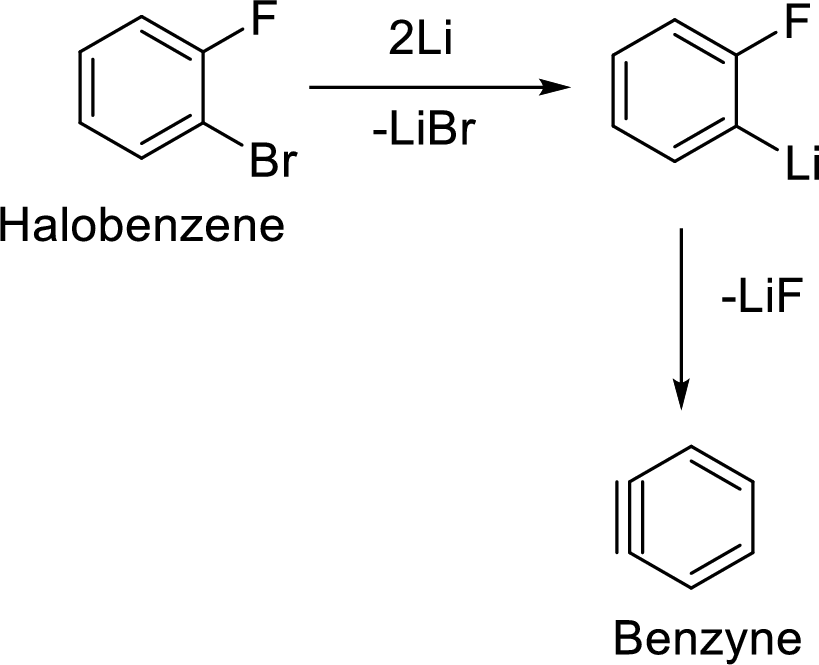
The elimination of two halogens from the halobenzene forms the most reactive neutral intermediate with triple bond which is known as Benzyne intermediate.
Diels-Alder reaction with Benzyne intermediate:
The reaction of Benzyne with furan serves as an example for this reaction:
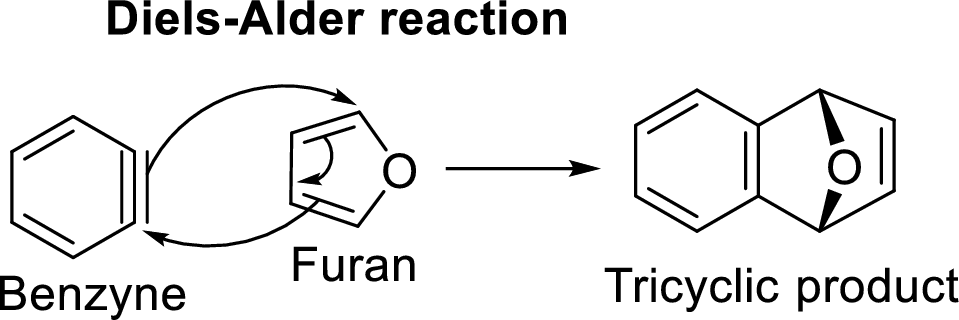
Benzyne is an extremely reactive species because of the presence of triple bond in it. It undergoes Diels-Alder reaction in which it acts as dienophile and reacts with furan which acts a diene. The resulted product will be a tricyclic product which is known as Diels-Alder adduct.
(b)
Interpretation: Using the given sources, the conversion of compound (A) into Tolciclate has to be shown.
Concept Introduction:
Acidic hydrolysis of ether:
Ether on hydrolysis gets converted into two moles of alcohols of same type in the case of symmetrical ether and of different types in the case of unsymmetrical ether. .
General scheme for symmetrical ether:
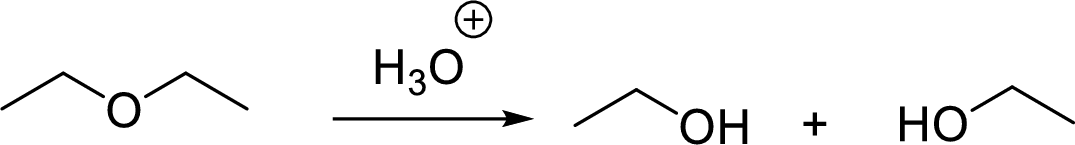
General scheme for unsymmetrical ether:
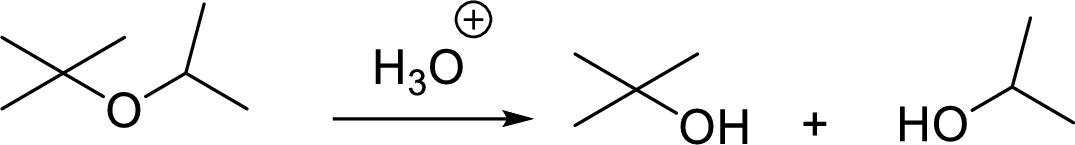
Tosylation reaction:
When an alcohol is treated with any tosyl chloride (methane sulfonyl chloride) it gets converted into tosylated product and this reaction is called as Tosylation reaction which is shown below:
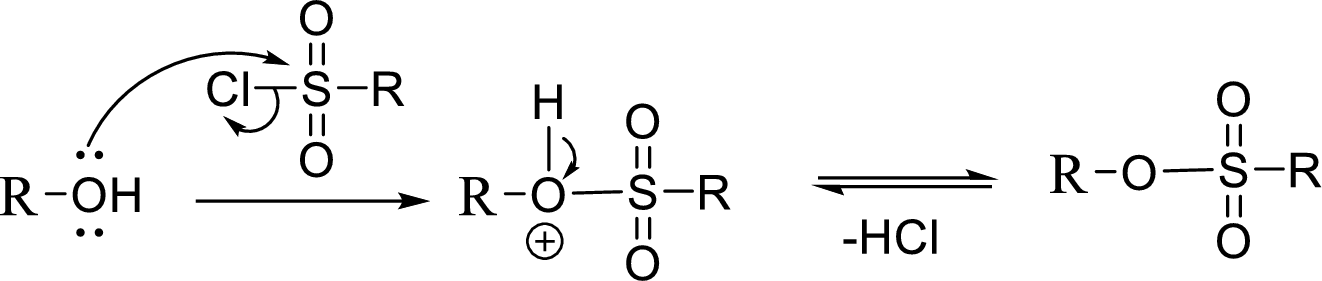
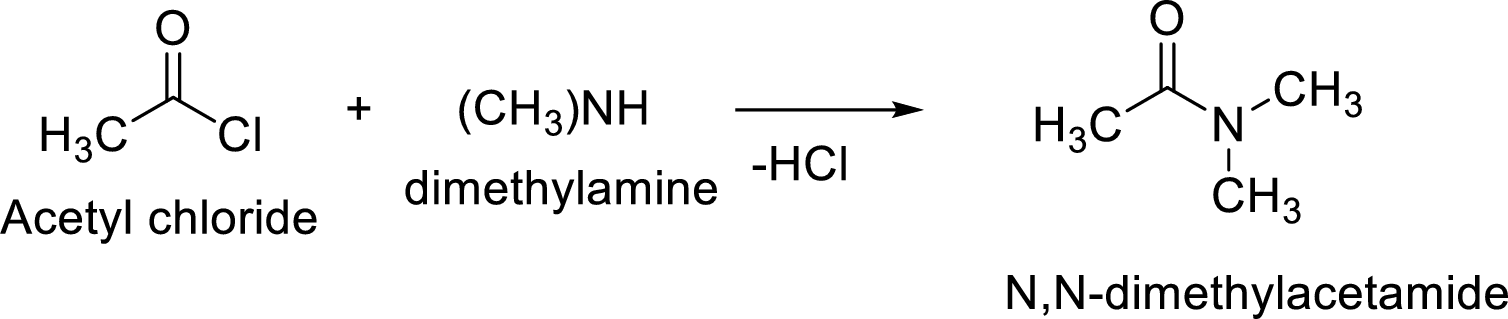
The reaction of acetyl chloride with
The acyl group of acetyl chloride is very reactive and the chloride group attached to it is a good leaving group. The nucleophile from amine attacks the carbonyl carbon of the acetylchloride to form N- methylacetamide. The chloride group leaves and gets attached to hydrogen of the amine and leaves as hydrochloric acid.
The reaction is:
Want to see the full answer?
Check out a sample textbook solution
Chapter 20 Solutions
ORGANIC CHEMISTRY-OWL V2 ACCESS
- Assign all the carbonsarrow_forward9 7 8 C 9 8 200 190 B 5 A -197.72 9 8 7 15 4 3 0: ང་ 200 190 180 147.52 134.98 170 160 150 140 130 120 110 100 90 90 OH 10 4 3 1 2 -143.04 140. 180 170 160 150 140 130 120 110 100 90 CI 3 5 1 2 141.89 140.07 200 190 180 170 160 150 140 130 120 110 100 ៖- 90 129. 126.25 80 70 60 -60 50 40 10 125.19 -129.21 80 70 3.0 20 20 -8 60 50 10 ppm -20 40 128.31 80 80 70 60 50 40 40 -70.27 3.0 20 10 ppm 00˚0-- 77.17 30 20 20 -45.36 10 ppm -0.00 26.48 22.32 ―30.10 ―-0.00arrow_forwardAssign all the carbonsarrow_forward
- C 5 4 3 CI 2 the Righ B A 5 4 3 The Lich. OH 10 4 5 3 1 LOOP- -147.52 T 77.17 -45.36 200 190 180 170 160 150 140 130 120 110 100 90 80 70 60 50 40 30 20 10 ppm B -126.25 77.03 200 190 180 170 160 150 140 130 120 110 100 90 80 70 60 50 40 30 20 10 ppm 200 190 180 170 160 150 140 130 120 110 100 90 80 TO LL <-50.00 70 60 50 40 30 20 10 ppm 45.06 30.18 -26.45 22.36 --0.00 45.07 7.5 1.93 2.05 -30.24 -22.36 C A 7 8 5 ° 4 3 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 ppm 9 8 5 4 3 ཡི་ OH 10 2 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 5 4 3 2 that th 7 I 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 115 2.21 4.00 1.0 ppm 6.96 2.76 5.01 1.0 ppm 6.30 1.00arrow_forwardCurved arrows were used to generate the significant resonance structure and labeled the most significant contribute. What are the errors in these resonance mechanisms. Draw out the correct resonance mechanisms with an brief explanation.arrow_forwardWhat are the: нсе * Moles of Hice while given: a) 10.0 ml 2.7M ? 6) 10.ome 12M ?arrow_forward
- You are asked to use curved arrows to generate the significant resonance structures for the following series of compounds and to label the most significant contributor. Identify the errors that would occur if you do not expand the Lewis structures or double-check the mechanisms. Also provide the correct answers.arrow_forwardhow to get limiting reactant and % yield based off this data Compound Mass 6) Volume(mL Ben zaphone-5008 ne Acetic Acid 1. Sam L 2-propanot 8.00 Benzopin- a col 030445 Benzopin a Colone 0.06743 Results Compound Melting Point (°c) Benzopin acol 172°c - 175.8 °c Benzoping to lone 1797-180.9arrow_forwardAssign ALL signals for the proton and carbon NMR spectra on the following pages.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning



