
Concept explainers
(a)
Interpretation:
The product with detailed mechanism for the reaction between methyl benzoate and
Concept introduction:
An ester can be converted to a primary alcohol by reacting with
Answer to Problem 20.46P
The product with detailed mechanism for the reaction between methyl benzoate and
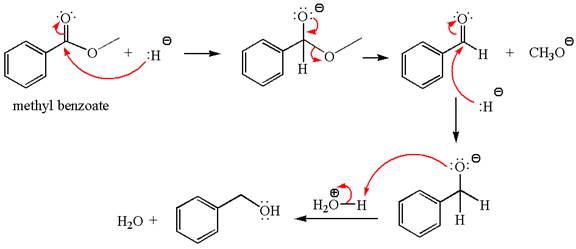
Explanation of Solution
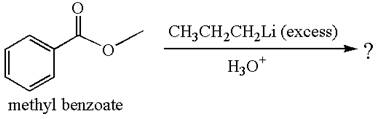
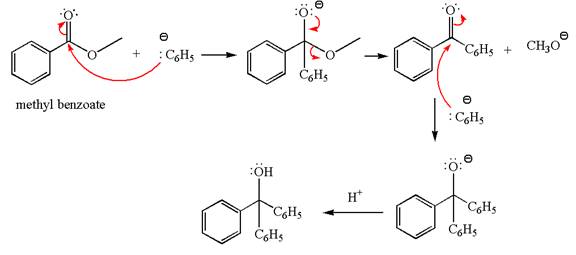
The equation for the reaction of methyl benzoate with
Methyl benzoate is an ester, which, on reaction with
The product with detailed mechanism for the given reaction is drawn based on the reactivity of
(b)
Interpretation:
The product with detailed mechanism for the reaction between methyl benzoate and
Concept introduction:
An ester can be converted to a tertiary alcohol by reacting it with
Answer to Problem 20.46P
The product with detailed mechanism for the reaction between methyl benzoate and
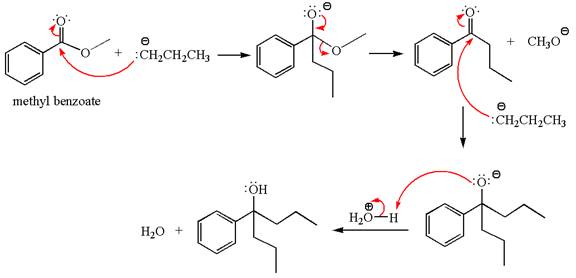
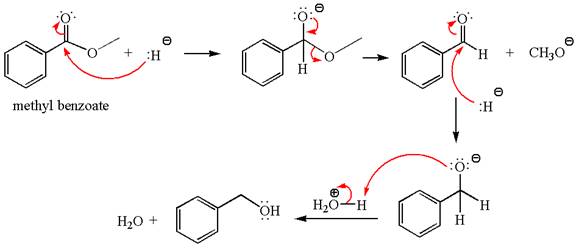
Explanation of Solution
The equation for the reaction of methyl benzoate with
Methyl benzoate is an ester, which, on reaction with
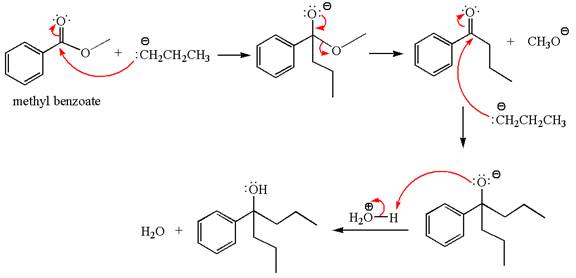
The product with detailed mechanism for the given reaction is drawn based on the reactivity of
(c)
Interpretation:
Whether methyl benzoate can react with
Concept introduction:
The reagent
Answer to Problem 20.46P
Mmethyl benzoate cannot react with
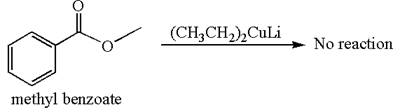
Explanation of Solution
The equation for the reaction of methyl benzoate with
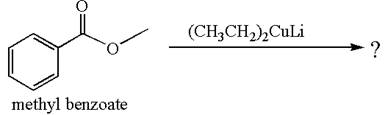
Methyl benzoate is an ester and
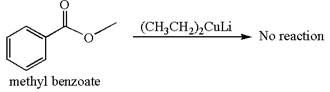
It is determined that no reaction occurs based on the reactivity of
(d)
Interpretation:
The product with detailed mechanism for the reaction between methyl benzoate and
Concept introduction:
An ester can be converted to a tertiary alcohol by reacting it with
Answer to Problem 20.46P
The product with detailed mechanism for the reaction between methyl benzoate and
Explanation of Solution
The equation for the reaction of methyl benzoate with
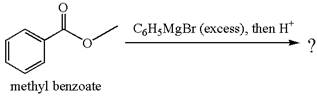
Methyl benzoate is an ester, which, on reaction with

The product with detailed mechanism for the given reaction is drawn based on the reactivity of
(e)
Interpretation:
The product with detailed mechanism for the reaction between methyl benzoate and
Concept introduction:
An ester can be reduced to aldehyde without reducing it further to alcohol using a specific reagent such as
Answer to Problem 20.46P
The product with detailed mechanism for the reaction between methyl benzoate and
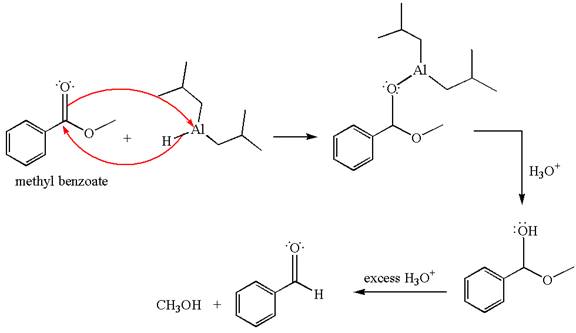
Explanation of Solution
The equation for the reaction of methyl benzoate with
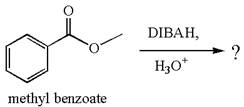
Methyl benzoate is an ester, which, on reaction with
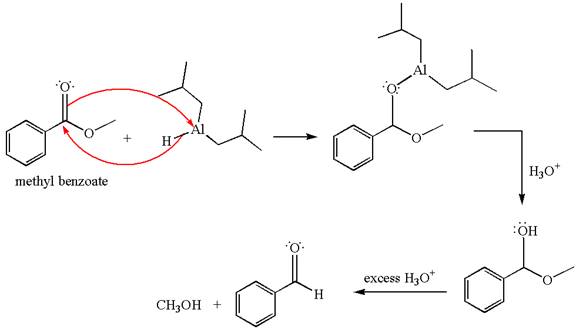
The product with detailed mechanism for the given reaction is drawn based on the reactivity of
Want to see more full solutions like this?
Chapter 20 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- please help i cant find the article to even startarrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardhelp with the rf values i am so confusedarrow_forward
- Predict the organic reactant of X and Y that are involved in the reaction below, and draw the skeletal ("line") structures of the missing organic reactant. Please include all steps & drawings & explanations.arrow_forwardPredict the major organic product for this reaction.arrow_forwardPredict the major organic product for this reaction.arrow_forward
- Predict the major organic product for this reaction.arrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardPlease provide the complete mechanism for the reaction below and include all appropriate arrows, formal charges, and intermediates. Please draw out the answerarrow_forward
