
In Figure P19.22, the change in internal energy of a gas that is taken from A to C along the blue path is +800 J. The work done on the gas along the red path ABC is −500 J. (a) How much energy must be added to the system by heat as it goes from A through B to C? (b) If the pressure at point A is five times that of point C, what is the work done on the system in going from C to D?
Figure P19.22
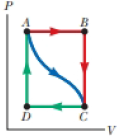
(c) What is the energy exchanged with the surroundings by heat as the gas goes from C to A along the green path? (d) If the change in internal energy in going from point D to point A is +500 J, how much energy must be added to the system by heat as it goes from point C to point D?
(a)
Answer to Problem 20.40P
Explanation of Solution
Given info: The change in internal energy of a gas which is taken along the blue path from A to C is
Write the equation of first law of thermodynamics.
Here,
Write the equation of conservation of energy.
Here,
Substitute
Substitute
Conclusion:
Therefore, the energy which must be added to the system by heat which goes from A through B to C is
(b)
Answer to Problem 20.40P
Explanation of Solution
Given info: The change in internal energy of a gas which is taken along the blue path from A to C is
Write the equation to calculate the work done on the system from C to D.
Here,
Here,
The pressure at point A is five times that of point C.
And the volume is,
Substitute
Substitute
Conclusion:
Therefore, the work done on the system from C to D if pressure at A is five times that of point C is
(c)
Answer to Problem 20.40P
Explanation of Solution
Given info: The change in internal energy of a gas which is taken along the blue path from A to C is
Write the equation of first law of thermodynamics.
Here,
Write the equation to calculate work done along the green path from C to A.
Here, the volume
Substitute 0 for
Substitute
Substitute
Conclusion:
Therefore, the energy exchanged with the surroundings by heat along the green path from C to A is
(d)
Answer to Problem 20.40P
is
Explanation of Solution
Given info: The change in internal energy of a gas which is taken along the blue path from A to C is
Write the equation of first law of thermodynamics.
Here,
Substitute
Conclusion:
Therefore, the energy which must be added to the system by heat which goes from C to D is
Want to see more full solutions like this?
Chapter 20 Solutions
EBK PHYSICS FOR SCIENTISTS AND ENGINEER
- Help me make a visualize experimental setup using a word document. For the theory below.arrow_forwardHow to solve this, given answerarrow_forwardThree point-like charges are placed at the corners of a square as shown in the figure, 28.0 cm on each side. Find the minimum amount of work required by an external force to move the charge q1 to infinity. Let q1=-2.10 μC, q2=+2.40 μС, q3=+3.60 μC.arrow_forward
- A point charge of -4.00 nC is at the origin, and a second point charge of 6.00 nC is on the x axis at x= 0.820 mm . Find the magnitude and direction of the electric field at each of the following points on the x axis. x2 = 19.0 cmarrow_forwardFour point-like charges are placed as shown in the figure, three of them are at the corners and one at the center of a square, 36.0 cm on each side. What is the electric potential at the empty corner? Let q1=q3=+26.0 µС, q2=-28.0 μC, and q4=-48.0μc Varrow_forwardPLS HELparrow_forward
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





