
Chemistry - Modified MasteringChemistry
7th Edition
ISBN: 9780133892321
Author: McMurry
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 20, Problem 20.142CP
Interpretation Introduction
Interpretation:
‘Crystal field energy level diagram for linear
Concept introduction:
There are five d orbitals namely
The two orbitals
Given: For linear
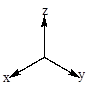
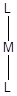
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
show the retrosynthesis of this
molecule step by step starting with
1,3-dimethoxy benzene
H3CO
OH
OH
OCH 3
Consider the reaction of a propanoate ester with hydroxide ion shown below. A
series of four alcohol leaving groups were tested to determine which would be the
best leaving group.
Based on the pKa values of the alcohols, predict which alcohol would produce the
fastest hydrolysis reaction.
HO
FOR
A
Alcohol I, pKa =16.0
B
Alcohol II, pKa =10.0
C
Alcohol III, pKa = 7.2
+ ROH
D
Alcohol IV, pKa = 6.6
Curved arrows are used to illustrate the flow of electrons. Using
the provided starting and product structures, draw the curved
electron-pushing arrows for the following reaction or
mechanistic step(s).
Be sure to account for all bond-breaking and bond-making
steps.
:0:
NaOH, H₂O
00:4
Na O
heat
NaO
Select to Add Arrows
Select to Add Arrows
:0:
Na
a
NaOH, H2O
:0:
NaOH,
H2O
heat
heat
Na
ONH
Select to Add Arrows
Chapter 20 Solutions
Chemistry - Modified MasteringChemistry
Ch. 20 - Prob. 20.1PCh. 20 - Prob. 20.2ACh. 20 - Prob. 20.3PCh. 20 - Prob. 20.4PCh. 20 - Prob. 20.5PCh. 20 - Prob. 20.6PCh. 20 - Prob. 20.7ACh. 20 - Prob. 20.8PCh. 20 - Prob. 20.9PCh. 20 - Prob. 20.10A
Ch. 20 - Prob. 20.11PCh. 20 - Prob. 20.12ACh. 20 - Prob. 20.13PCh. 20 - Prob. 20.14ACh. 20 - Prob. 20.15PCh. 20 - Prob. 20.16ACh. 20 - Prob. 20.17PCh. 20 - Prob. 20.18ACh. 20 - Prob. 20.19PCh. 20 - Prob. 20.20PCh. 20 - Prob. 20.21ACh. 20 - Prob. 20.22PCh. 20 - Prob. 20.23ACh. 20 - Prob. 20.24PCh. 20 - Prob. 20.25ACh. 20 - Prob. 20.26PCh. 20 - Prob. 20.27PCh. 20 - Prob. 20.28PCh. 20 - Prob. 20.29PCh. 20 - Prob. 20.30CPCh. 20 - Prob. 20.31CPCh. 20 - Prob. 20.32CPCh. 20 - Prob. 20.33CPCh. 20 - Prob. 20.34CPCh. 20 - Prob. 20.35CPCh. 20 - Prob. 20.36CPCh. 20 - Prob. 20.37CPCh. 20 - Prob. 20.38CPCh. 20 - Prob. 20.39CPCh. 20 - Prob. 20.40SPCh. 20 - Prob. 20.41SPCh. 20 - Prob. 20.42SPCh. 20 - Prob. 20.43SPCh. 20 - Titanium, used to make jet aircraft engines, is...Ch. 20 - Prob. 20.45SPCh. 20 - Prob. 20.46SPCh. 20 - Prob. 20.47SPCh. 20 - Prob. 20.48SPCh. 20 - Prob. 20.49SPCh. 20 - Prob. 20.50SPCh. 20 - Prob. 20.51SPCh. 20 - Prob. 20.52SPCh. 20 - Prob. 20.53SPCh. 20 - Prob. 20.54SPCh. 20 - Prob. 20.55SPCh. 20 - Prob. 20.56SPCh. 20 - Prob. 20.57SPCh. 20 - Prob. 20.58SPCh. 20 - Prob. 20.59SPCh. 20 - Prob. 20.60SPCh. 20 - Prob. 20.61SPCh. 20 - Prob. 20.62SPCh. 20 - Prob. 20.63SPCh. 20 - Prob. 20.64SPCh. 20 - Prob. 20.65SPCh. 20 - Prob. 20.66SPCh. 20 - Prob. 20.67SPCh. 20 - Prob. 20.68SPCh. 20 - Prob. 20.69SPCh. 20 - Prob. 20.70SPCh. 20 - Prob. 20.71SPCh. 20 - Prob. 20.72SPCh. 20 - Prob. 20.73SPCh. 20 - Prob. 20.74SPCh. 20 - Prob. 20.75SPCh. 20 - Prob. 20.76SPCh. 20 - Prob. 20.77SPCh. 20 - Prob. 20.78SPCh. 20 - Prob. 20.79SPCh. 20 - Prob. 20.80SPCh. 20 - Prob. 20.81SPCh. 20 - Prob. 20.82SPCh. 20 - Prob. 20.83SPCh. 20 - Prob. 20.84SPCh. 20 - Prob. 20.85SPCh. 20 - Prob. 20.86SPCh. 20 - Prob. 20.87SPCh. 20 - Prob. 20.88SPCh. 20 - Prob. 20.89SPCh. 20 - Prob. 20.90SPCh. 20 - Prob. 20.91SPCh. 20 - Prob. 20.92SPCh. 20 - Prob. 20.93SPCh. 20 - Prob. 20.94SPCh. 20 - Prob. 20.95SPCh. 20 - Prob. 20.96SPCh. 20 - Prob. 20.97SPCh. 20 - Prob. 20.98SPCh. 20 - Prob. 20.99SPCh. 20 - Prob. 20.100SPCh. 20 - Prob. 20.101SPCh. 20 - Prob. 20.102SPCh. 20 - Prob. 20.103SPCh. 20 - Prob. 20.104SPCh. 20 - Prob. 20.105SPCh. 20 - Prob. 20.106SPCh. 20 - Prob. 20.107SPCh. 20 - Prob. 20.108SPCh. 20 - Prob. 20.109SPCh. 20 - Prob. 20.110SPCh. 20 - Prob. 20.111SPCh. 20 - Prob. 20.112SPCh. 20 - Prob. 20.113SPCh. 20 - Prob. 20.114SPCh. 20 - Prob. 20.115SPCh. 20 - Prob. 20.116SPCh. 20 - Prob. 20.117SPCh. 20 - Prob. 20.118SPCh. 20 - Prob. 20.119SPCh. 20 - Prob. 20.120SPCh. 20 - Prob. 20.121SPCh. 20 - Prob. 20.122SPCh. 20 - Prob. 20.123SPCh. 20 - Prob. 20.124CPCh. 20 - Prob. 20.125CPCh. 20 - Prob. 20.126CPCh. 20 - Prob. 20.127CPCh. 20 - Prob. 20.128CPCh. 20 - Prob. 20.129CPCh. 20 - Prob. 20.130CPCh. 20 - Prob. 20.131CPCh. 20 - Prob. 20.132CPCh. 20 - Prob. 20.133CPCh. 20 - Prob. 20.134CPCh. 20 - Prob. 20.135CPCh. 20 - Prob. 20.136CPCh. 20 - Prob. 20.137CPCh. 20 - Prob. 20.138CPCh. 20 - Prob. 20.139CPCh. 20 - Prob. 20.140CPCh. 20 - Prob. 20.141CPCh. 20 - Prob. 20.142CPCh. 20 - Prob. 20.143CPCh. 20 - Prob. 20.144CPCh. 20 - Prob. 20.145CPCh. 20 - Prob. 20.146CPCh. 20 - Prob. 20.147CPCh. 20 - Prob. 20.148CPCh. 20 - Prob. 20.149MPCh. 20 - Prob. 20.150MPCh. 20 - Prob. 20.151MPCh. 20 - Prob. 20.152MPCh. 20 - Prob. 20.153MPCh. 20 - Prob. 20.154MPCh. 20 - Prob. 20.155MPCh. 20 - Prob. 20.156MPCh. 20 - Prob. 20.157MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. H CH3NH3+ :0: :0: HO CH3NH2 HH iSelect to Add Arrows i Select to Add Arrows i HH CH3NH3+ CH3NH2 Select to Add Arrows i CH3NH3 CH3NH2 ايكدا HH Select to Add Arrowsarrow_forwardThe reaction is carried out with gases: A → B + C at 300 K. The total pressure is measured as a function of time (table). If the reaction order is 2, calculate the rate or kinetic constant k (in mol-1 L s¹) Ptotal (atm) 492 676 760 808 861 t(s) 0 600 1200 1800 3000arrow_forwardcan someone give a description of this NMR including whether its a triplt singlet doublet where the peak is around at ppm and what functional group it representsarrow_forward
- 1. Determine the relationship between the following molecules as identical, diastereomers, or enantiomers (6 points, 2 points each). OH OH OH A-A OH HOT HO- ACHN and HO- ACHN OH HO HO ° OH and OH OH SH and ...SHarrow_forward20,0 Complete the electron pushing mechanism to y drawing the necomery unicaciones and carved on for Step 1: Add curved arms for the tint step, traiment with NalilĻ. The Nation 458 Step 2: Added for the second step, inalment with), how the "counterion bar Step 3: Daw the products of the last simplom organic and one incoganic spacient, including all nonbondingarrow_forwardplease provide the structure for this problem, thank you!arrow_forward
- Draw the Fischer projection from the skeletal structure shown below. HO OH OH OH OH H Q Drawing Atoms, Bonds and Rings Charges I ☐ T HO H H OH HO I CH2OH H OH Drag H OH -CH2OH CHO -COOH Undo Reset Remove Donearrow_forwardplease provide the structure for this problem, thank youarrow_forwardpresented by Morallen Lig Intermine the hand product for the given mution by adding atoms, bonds, nonhonding diarion panda скуль Step 3: Comp the draw the product Step 2: Agama workup Compithe 429 ملولةarrow_forward
- Reaction A 0,0arrow_forwardpresented by Morillon Leaning Predict the organic product for the min кусур HSC Adithane carved arnown to come than that to the condon slchroruis in acid in in aquishri with ноюarrow_forward6.15PM Sun Mar 30 K Draw the major product of this reaction. Include any relevant stereochemistry. Ignore inorganic byproducts. Problem 1 of O H [PhзPCH2CH3]*C|¯ NaH Drawing > Q Atoms, Bonds and Draw or tap a nearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
The Bohr Model of the atom and Atomic Emission Spectra: Atomic Structure tutorial | Crash Chemistry; Author: Crash Chemistry Academy;https://www.youtube.com/watch?v=apuWi_Fbtys;License: Standard YouTube License, CC-BY