
Concept explainers
An
- (a) Identify the element and give its symbol.
- (b) Give the element’s
atomic number . - (c) Give the mass number of the isotope.
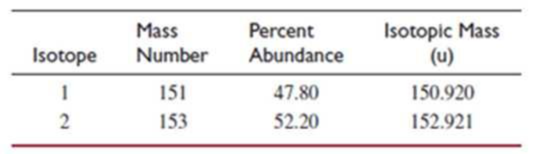
- (d) This element has two naturally occurring isotopes. Given the information in the table, calculate the atomic weight of the element.
- (e) In which region of the periodic table is the element found? Explain your answer.
- (f) Is the element a metal, metalloid, or nonmetal? Explain your answer.
- (g) This element, used in compact fluorescent light bulbs and computer screens, has an atomic radius of 180 pm. Calculate how long the chain of atoms would be if all the atoms in a 1.25-mg sample of this element were put into a row.
(a)
Interpretation:
The isotope of an element contains 63 protons and 91 neutrons. The element has to be identified and the symbol of the element has to be given.
Explanation of Solution
Number of protons of the element is 63.
Number of neutrons of the element is 91.
The atomic number is same as that of number of protons, 63. Also the number of protons is same as that number of electrons in an uncharged atom. The element is Europium with a symbol of
(b)
Interpretation:
The atomic number of element with 63 protons and 91 neutrons has to be given.
Explanation of Solution
Number of protons of the element is 63.
The atomic number is same as that of number of protons, 63.
Atomic number
(c)
Interpretation:
The mass number of the isotope with 63 protons and 91 neutrons has to be given.
Explanation of Solution
Number of protons of the element is 63.
Number of neutrons of the element is 91.
Atomic number
Mass number is the sum of atomic number of the element and number of neutrons.
Isotope’s mass number
(d)
Interpretation:
The element has two naturally occurring isotopes. From the table given the atomic weight of the element has to be calculated.
Explanation of Solution
Every 10000 atoms of the element contain 4780 atoms of the
(e)
Interpretation:
The region in which the element is found in periodic table has to be explained.
Explanation of Solution
The element is found in the lanthanide series because it shows up in the row labeled “Lanthanides”.
(f)
Interpretation:
The given element is a metal, metalloid or non-metal has to be found and the answer has to be explained.
Explanation of Solution
The element Europium is a transition metal.
(g)
Interpretation:
The element given has an atomic radius of
Explanation of Solution
The atomic radius is
Want to see more full solutions like this?
Chapter 2 Solutions
Bundle: Chemistry: The Molecular Science, 5th, Loose-Leaf + OWLv2 with Quick Prep 24-Months Printed Access Card
- Please do these questions within the SCH4U course please with full steps since I am still unsure how to format my answers! Thank you so much.arrow_forwardWhen two solutions, one of 0.1 M KCl (I) and the other of 0.1 M MCl (II), are brought into contact by a membrane. The cation M cannot cross the membrane. At equilibrium, x moles of K+ will have passed from solution (I) to (II). To maintain the neutrality of the two solutions, x moles of Cl- will also have to pass from I to II. Explain this equality: (0.1 - x)/x = (0.1 + x)/(0.1 - x)arrow_forwardCalculate the variation in the potential of the Pt/MnO4-, Mn2+ pair with pH, indicating the value of the standard potential. Data: E0 = 1.12.arrow_forward
- Given the cell: Pt l H2(g) l dis X:KCl (sat) l Hg2Cl2(s) l Hg l Pt. Calculate the emf of the cell as a function of pH.arrow_forwardThe decimolar calomel electrode has a potential of 0.3335 V at 25°C compared to the standard hydrogen electrode. If the standard reduction potential of Hg22+ is 0.7973 V and the solubility product of Hg2Cl2 is 1.2x 10-18, find the activity of the chlorine ion at this electrode.Data: R = 8.314 J K-1 mol-1, F = 96485 C mol-1, T = 298.15 K.arrow_forward2. Add the following group of numbers using the correct number of significant figures for the answer. Show work to earn full credit such as rounding off the answer to the correct number of significant figures. Replace the question marks with the calculated answers or write the calculated answers near the question marks. 10916.345 37.40832 5.4043 3.94 + 0.0426 ? (7 significant figures)arrow_forward
- The emf at 25°C of the cell: Pt l H2(g) l dis X:KCl (sat) l Hg2Cl2(s) l Hg l Pt was 612 mV. When solution X was replaced by normal phosphate buffer solution with a pH of 6.86, the emf was 741 mV. Calculate the pH of solution X.arrow_forwardIndicate how to calculate the potential E of the reaction Hg2Cl2(s) + 2e ⇄ 2Hg + 2Cl- as a function of the concentration of Cl- ions. Data: the solubility product of Hg2Cl2.arrow_forwardHow can Beer’s Law be used to determine the concentration in a selected food sample. Provide an in-depth discussion and examples of this.arrow_forward
- b) H3C- H3C Me CH 3 I HN Me H+arrow_forwardUsing Luther's rule, determine the reference potentials of the electrodes corresponding to the low stability systems Co³+/Co and Cr²+/Cr from the data in the table. Electrodo ΕΝ Co²+/Co Co3+/Co²+ -0,28 +1,808 Cr³+ / Cr -0,508 Cr3+ / Cr²+ -0,41arrow_forwardThe molecule PYRIDINE, 6tt electrons and is there pore aromuntre and is Assigned the Following structure contenus Since aromatk moleculey undergo electrophilic allomatic substitution, Pyridine should undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this roaction Based upon the reaction the reaction mechanism determine which of these producty would be the major Product of the hegetionarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





