
Concept explainers
One structure for the acetoxonium ion is
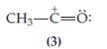
Clearly, the receptor is the positively charged
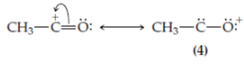
structure (4) is obtained in which the oxygen possesses a formal positive charge but, in addition, both the oxygen and the carbon atom lack a stable octet. This structure will be very unstable. Structure (4), despite the fact that it can be generated by properly pushing electrons, is not included in the resonance hybrid for the acetoxonium ion. If, on the other hand, the unshared electrons on the oxygen are pushed, a more acceptable structure is obtained, namely,
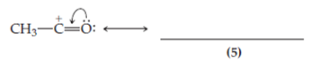
In structure (5) the oxygen atom possesses a formal positive charge, and both the carbon and the oxygen atom have a stable octet. Structure (5) is included in the resonance hybrid.
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
Pushing Electrons
- Check Consider the 13 C NMR spectrum below. 140 120 100 80 60 60 PPM 40 20 0 The spectrum belongs to which one of the following constitutional isomers of the compound C 10H14? Select the single best answer. ✓ Save © 2025 McGraw Hill LLC. All Rights Reserved.arrow_forwardThe structure of compound 1,1,2-trichloropropane is given below. Cl Cl Cl 1 How many signals would you expect to find in the 'H NMR spectrum of 1,1,2-trichloropropane? ×arrow_forward1, How many signals do you expect in the H NMR spectrum for this molecule? Write the answer below. Also, in each of the drawing areas below is a copy of the molecule, with Hs shown. In each copy, one of the H atoms is colored red. Highlight in red all other H atoms that would contribute to the same signal as the H already highlighted red. Note for advanced students: In this question, any multiplet is counted as one signal. Number of signals in the 'H NMR spectrum. For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. No additional Hs to color in top molecule For the molecule in the bottom drawing area, highlight in red any other H atoms that will contribute. to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. No additional Hs to color in bottom molecule Check…arrow_forward
- Incorrect Row 2: Your answer is incorrect. Consider this molecule: How many H atoms are in this molecule? 22 How many different signals could be found in its 'H NMR spectrum? 12 Note: A multiplet is considered one signal.arrow_forward13 How many signals would you expect to see in the Check O signal(s) X § 'C NMR spectrum for the following compound? © 2025 McGraw Hillarrow_forward13 Consider the "C NMR spectrum below. 140 120 100 80 60 40 20 20 PPM 0 The spectrum belongs to which one of the following constitutional isomers of the compound C,H12? Select the single best answer. Check ✓ G Save For Later 2025 McGraw Hill LLC. All Rights Reserved. Terms of Usearrow_forward
- The structure of compound 1,3,5-trimethylbenzene (mesitylene) is given below. How many signals would you expect to find in the 'H NMR spectrum of 1,3,5-trimethylbenzene (mesitylene)? Check ×arrow_forward1 How many signals do you expect in the 'H NMR spectrum for this molecule? CI CI Cl Write the answer in the table below. Also, in each of the drawing areas below is a copy of the molecule, with H atoms shown. In each copy, one of the H atoms is highlighted red. Highlight in red all other H atoms that would contribute to the same signal as the H already highlighted red. Note for advanced students: Remember, a multiplet is considered one signal in the 'H NMR spectrum. 1 Number of signals in the 'H NMR spectrum. ☐ For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. No additional H atoms to highlight in top molecule For the molecule in the bottom drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at…arrow_forwardwrtie the balanced equation and find the E° when the following half- reactions are combined Zn2+(aq) + 2e---> Zn(s) E°= -0.763V Ag+(aq) + e---> Ag (s) E°=+0.799Varrow_forward

 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

