
CHEMISTRY+CHEM...HYBRID ED.(LL)>CUSTOM<
9th Edition
ISBN: 9781305020788
Author: John C.Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: CENGAGE C
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 77PS
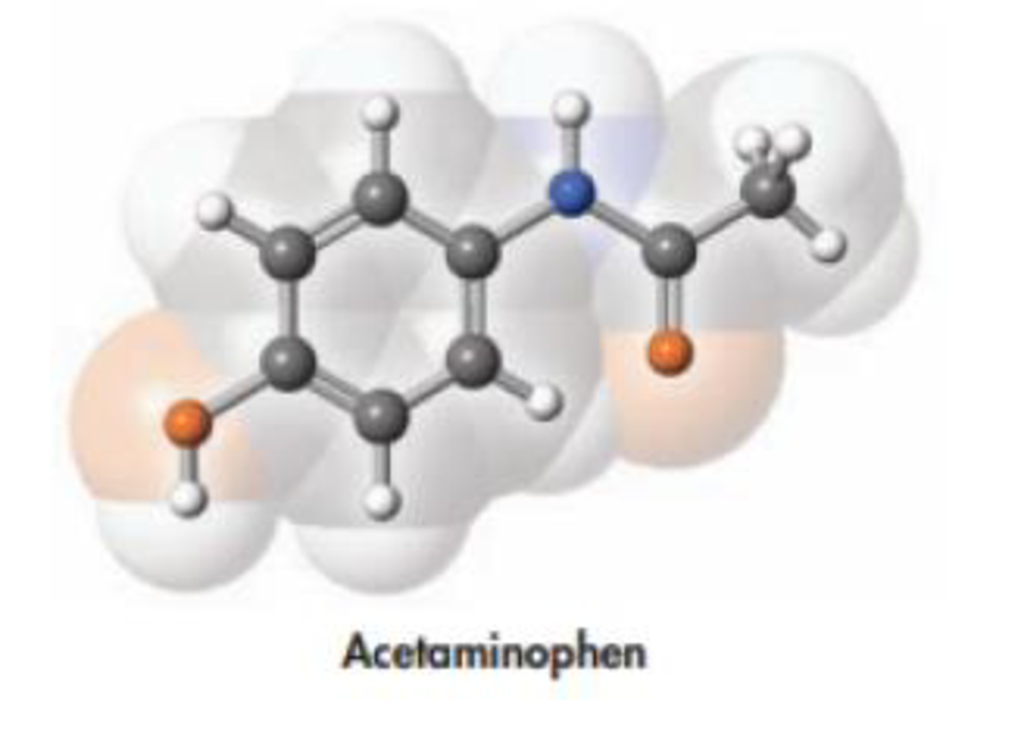
Acetaminophen, whose structure is drawn below, is the active ingredient in some nonprescription pain killers. The recommended dose for an adult is two 500-mg caplets. How many molecules make up one dose of this drug?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Vnk the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest bolling
point, choose 2 next to the substance with the next highest boiling point, and so on.
substance
C
D
chemical symbol,
chemical formula
or Lewis structure.
CH,-N-CH,
CH,
H
H 10: H
C-C-H
H H H
Cale
H 10:
H-C-C-N-CH,
Bri
CH,
boiling point
(C)
Сен
(C) B
(Choose
Please help me find the 1/Time, Log [I^-] Log [S2O8^2-], Log(time) on the data table. With calculation steps. And the average for runs 1a-1b. Please help me thanks in advance. Will up vote!
Q1: Answer the questions for the reaction below:
..!! Br
OH
a) Predict the product(s) of the reaction.
b) Is the substrate optically active? Are the product(s) optically active as a mix?
c) Draw the curved arrow mechanism for the reaction.
d) What happens to the SN1 reaction rate in each of these instances:
1. Change the substrate to
Br
"CI
2. Change the substrate to
3. Change the solvent from 100% CH3CH2OH to 10% CH3CH2OH + 90% DMF
4. Increase the substrate concentration by 3-fold.
Chapter 2 Solutions
CHEMISTRY+CHEM...HYBRID ED.(LL)>CUSTOM<
Ch. 2.2 - 1. What is the mass of an iron atom with 30...Ch. 2.2 - 1. The mass of an atom of manganese is 54.9380 u....Ch. 2.2 - An atom contains 12 neutrons and has a mass number...Ch. 2.3 - 1. Silver has two isotopes, one with 60 neutrons...Ch. 2.3 - 2. A naturally occurring sample of argon contains...Ch. 2.4 - Verify that the atomic weight of chlorine is...Ch. 2.4 - Neon has three stable isotopes, one with a small...Ch. 2.4 - 1. Which is the more abundant isotope of copper,...Ch. 2.4 - 2. Which of the following is closest to the...Ch. 2.4 - Prob. 3RC
Ch. 2.5 - Prob. 1QCh. 2.5 - Prob. 2QCh. 2.5 - Which of the following elements is a metalloid?...Ch. 2.5 - Prob. 2RCCh. 2.5 - Prob. 3RCCh. 2.5 - Prob. 4RCCh. 2.6 - Prob. 1RCCh. 2.7 - Give the number and identity of the constituent...Ch. 2.7 - (a) Write the formulas of all neutral ionic...Ch. 2.7 - Prob. 1RCCh. 2.7 - Prob. 2RCCh. 2.7 - Prob. 3RCCh. 2.7 - The formula of barium acetate is (a) Ba(CH3CO2)2...Ch. 2.7 - 5. The name of the compound with the formula V2O3...Ch. 2.7 - Which should have the higher melting point, MgO or...Ch. 2.8 - Prob. 1RCCh. 2.8 - Prob. 2RCCh. 2.8 - Prob. 3RCCh. 2.8 - Prob. 4RCCh. 2.9 - The density of gold, Au, is 19.32 g/cm3. What is...Ch. 2.9 - If you have 454 g of citric acid (H3C6H5O7), what...Ch. 2.9 - Prob. 1RCCh. 2.9 - Which of the following contains the largest number...Ch. 2.9 - Which of the following has the largest mass? (a)...Ch. 2.9 - Prob. 4RCCh. 2.9 - Prob. 5RCCh. 2.10 - 1. Express the composition of ammonium carbonate,...Ch. 2.10 - 1. What is the empirical formula of naphthalene,...Ch. 2.10 - Gallium oxide, GaxOy forms when gallium is combine...Ch. 2.10 - Hydrated nickel(II) chloride is a beautiful green,...Ch. 2.10 - Prob. 1QCh. 2.10 - Salvarsan was long thought to be a single...Ch. 2.10 - To determine the density of atmospheric nitrogen....Ch. 2.10 - The density of a mixture of gases may be...Ch. 2.10 - Atmospheric argon is a mixture of three stable...Ch. 2.10 - Given that the density of argon is 1.78 g/L under...Ch. 2.10 - 1. Which of the following hydrocarbons has the...Ch. 2.10 - 2. An organic compound has an empirical formula...Ch. 2.10 - Eugenol is the major component in oil of cloves....Ch. 2.10 - 4. Epsom salt is MgSO4 · 7H2O. When heated to 70...Ch. 2 - Prob. 1PSCh. 2 - Define mass number. What is the difference between...Ch. 2 - An atom has a very small nucleus surrounded by an...Ch. 2 - A gold atom has a radius of 145 pm. If you could...Ch. 2 - Give the complete symbol(ZAX), including atomic...Ch. 2 - Give the complete symbol(ZAX), including atomic...Ch. 2 - Prob. 7PSCh. 2 - Atomic structure. (a) The synthetic radioactive...Ch. 2 - Prob. 9PSCh. 2 - In 1886 Eugene Goldstein observed positively...Ch. 2 - Marie Curie was born in Poland but studied and...Ch. 2 - Prob. 12PSCh. 2 - The mass of an 16 O atom is 15.995 u. What is its...Ch. 2 - What is the mass of one 16O atom, in grams? (The...Ch. 2 - Cobalt has three radioactive isotopes used in...Ch. 2 - Naturally occurring silver exists as two isotopes...Ch. 2 - Name and describe the composition of the three...Ch. 2 - Which of the following are isotopes of element X,...Ch. 2 - Thallium has two stable isotopes, 203TIand 205Tl....Ch. 2 - Strontium has four stable isotopes. Strontium-84...Ch. 2 - Verify that the atomic weight of lithium is 6.94,...Ch. 2 - Verify that the atomic weight of magnesium is...Ch. 2 - Gallium has two naturally occurring isotopes, 69Ga...Ch. 2 - Europium has two stable isotopes, 151Eu and 153Eu,...Ch. 2 - Titanium and thallium have symbols that are easily...Ch. 2 - In Groups 4A-6A, there are several elements whose...Ch. 2 - How many periods of the periodic table have 8...Ch. 2 - Prob. 28PSCh. 2 - Prob. 29PSCh. 2 - Prob. 30PSCh. 2 - Classify the following elements as metals,...Ch. 2 - Prob. 32PSCh. 2 - Prob. 33PSCh. 2 - Prob. 34PSCh. 2 - What is the charge on the common monatomic ions of...Ch. 2 - What is the charge on the common monatomic ions of...Ch. 2 - Prob. 37PSCh. 2 - Prob. 38PSCh. 2 - When a potassium atom becomes a monatomic ion, how...Ch. 2 - When oxygen and sulfur atoms become monatomic...Ch. 2 - Prob. 41PSCh. 2 - Prob. 42PSCh. 2 - Give the formula and the number of each ion that...Ch. 2 - Give the formula and the number of each ion that...Ch. 2 - Prob. 45PSCh. 2 - Prob. 46PSCh. 2 - Prob. 47PSCh. 2 - Prob. 48PSCh. 2 - Prob. 49PSCh. 2 - Prob. 50PSCh. 2 - Prob. 51PSCh. 2 - Prob. 52PSCh. 2 - Write the formulas for the four ionic compounds...Ch. 2 - Write the formulas for the four ionic compounds...Ch. 2 - Sodium ions, Na+, form ionic compounds with...Ch. 2 - Consider the two ionic compounds NaCl and CaO. In...Ch. 2 - Prob. 57PSCh. 2 - Name each of the following binary, nonionic...Ch. 2 - Prob. 59PSCh. 2 - Prob. 60PSCh. 2 - Calculate the mass, in grams, of each the...Ch. 2 - Calculate the mass, in grams, of each the...Ch. 2 - Calculate the amount (moles) represented by each...Ch. 2 - Calculate the amount (moles) represented by each...Ch. 2 - You are given 1.0-g samples of He, Fe, Li, Si, and...Ch. 2 - You are given 0.10-g samples of K, Mo, Cr, and Al....Ch. 2 - Analysis of a 10.0-g sample of apatite (a major...Ch. 2 - A semiconducting material is composed of 52 g of...Ch. 2 - Calculate the molar mass of each of the following...Ch. 2 - Calculate the molar mass of each of the following...Ch. 2 - Calculate the molar mass of each hydrated...Ch. 2 - Prob. 72PSCh. 2 - What mass is represented by 0.0255 mol of each of...Ch. 2 - Assume you have 0.123 mol of each of the following...Ch. 2 - Sulfur trioxide, SO3, is made industrially in...Ch. 2 - How many ammonium ions and how many sulfate ions...Ch. 2 - Acetaminophen, whose structure is drawn below, is...Ch. 2 - An Alka-Seltzer tablet contains 324 mg of aspirin...Ch. 2 - Calculate the mass percent of each element in the...Ch. 2 - Calculate the mass percent of each element in the...Ch. 2 - Calculate the mass percent of copper in CuS,...Ch. 2 - Calculate the mass percent of titanium in the...Ch. 2 - Succinic acid occurs in fungi and lichens. Its...Ch. 2 - An organic compound has the empirical formula...Ch. 2 - Prob. 85PSCh. 2 - Complete the following table:Ch. 2 - Acetylene is a colorless gas used as a fuel in...Ch. 2 - A large family of boron-hydrogen compounds has the...Ch. 2 - Cumene, a hydrocarbon, is a compound composed only...Ch. 2 - In 2006, a Russian team discovered an interesting...Ch. 2 - Mandelic acid is an organic acid composed of...Ch. 2 - Nicotine, a poisonous compound found in tobacco...Ch. 2 - A compound containing xenon and fluorine was...Ch. 2 - Elemental sulfur (1.256 g) is combined with...Ch. 2 - Epsom salt is used in tanning leather and in...Ch. 2 - You combine 1.25 g of germanium, Ge, with excess...Ch. 2 - Fill in the blanks in the table (one column per...Ch. 2 - Potassium has three naturally occurring isotopes...Ch. 2 - Crossword Puzzle: In the 2 2 box shown here, each...Ch. 2 - The following chart shows a general decline in...Ch. 2 - Copper atoms. (a) What is the average mass of one...Ch. 2 - Prob. 102GQCh. 2 - Prob. 103GQCh. 2 - Identify two nonmetallic elements that have...Ch. 2 - Prob. 105GQCh. 2 - Prob. 106GQCh. 2 - Prob. 107GQCh. 2 - When a sample of phosphorus burns in air, the...Ch. 2 - Although carbon-12 is now used as the standard for...Ch. 2 - A reagent occasionally used in chemical synthesis...Ch. 2 - Prob. 111GQCh. 2 - Prob. 112GQCh. 2 - Which of the following compounds has the highest...Ch. 2 - Which of the following samples has the largest...Ch. 2 - The structure of one of the bases in DNA, adenine,...Ch. 2 - Prob. 116GQCh. 2 - A drop of water has a volume of about 0.050 mL....Ch. 2 - Capsaicin, the compound that gives the hot taste...Ch. 2 - Prob. 119GQCh. 2 - Write the molecular formula and calculate the...Ch. 2 - Malic acid, an organic acid found in apples,...Ch. 2 - Your doctor has diagnosed you as being anemicthat...Ch. 2 - A compound composed of iron and carbon monoxide,...Ch. 2 - Ma huang, an extract from the ephedra species of...Ch. 2 - Saccharin, a molecular model of which is shown...Ch. 2 - Prob. 126GQCh. 2 - Write the formula for each of the following pounds...Ch. 2 - Complete the table by placing symbols, formulas,...Ch. 2 - Empirical and molecular formulas. (a)...Ch. 2 - Cacodyl, a compound containing arsenic, was...Ch. 2 - The action of bacteria on meat and fish produces a...Ch. 2 - In the laboratory you combine 0.125 g of nickel...Ch. 2 - A compound called MMT was once used to boost the...Ch. 2 - Elemental phosphorus is made by heating calcium...Ch. 2 - Chromium is obtained by heating chromium(III)...Ch. 2 - Stibnite, Sb2S3, is a dark gray mineral from which...Ch. 2 - Direct reaction of iodine (I2) and chlorine (Cl2)...Ch. 2 - In a reaction, 2.04 g of vanadium combined with...Ch. 2 - Iron pyrite, often called fools gold, has the...Ch. 2 - Which of the following statements about 57.1 g of...Ch. 2 - The formula of barium molybdate is BaMoO4. Which...Ch. 2 - A metal M forms a compound with the formula MCl4....Ch. 2 - Pepto-Bismol, which can help provide relief for an...Ch. 2 - The weight percent of oxygen in an oxide that has...Ch. 2 - The mass of 2.50 mol of a compound with the...Ch. 2 - The elements A and Z combine to produce two...Ch. 2 - Polystyrene can be prepared by heating styrene...Ch. 2 - A sample of hemoglobin is found to be 0.335% iron....Ch. 2 - Consider an atom of 64Zn. (a) Calculate the...Ch. 2 - Estimating the radius of a lead atom. (a) You are...Ch. 2 - A piece of nickel foil, 0.550 mm thick and 1.25 cm...Ch. 2 - Uranium is used as a fuel, primarily in the form...Ch. 2 - In an experiment, you need 0.125 mol of sodium...Ch. 2 - Mass spectrometric analysis showed that there are...Ch. 2 - The mass spectrum of CH3Cl is illustrated here....Ch. 2 - Prob. 156GQCh. 2 - If Epsom salt, MgSO4 x H2O, is heated to 250 C,...Ch. 2 - The alum used in cooking is potassium aluminum...Ch. 2 - Tin metal (Sn) and purple iodine (I2) combine to...Ch. 2 - When analyzed, an unknown compound gave these...Ch. 2 - Two general chemistry students working together in...Ch. 2 - To find the empirical formula of tin oxide, you...Ch. 2 - Prob. 163SCQCh. 2 - Prob. 164SCQCh. 2 - The photo here depicts what happens when a coil of...Ch. 2 - A jar contains some number of jelly beans. To find...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Experiment 27 hates & Mechanisms of Reations Method I visual Clock Reaction A. Concentration effects on reaction Rates Iodine Run [I] mol/L [S₂082] | Time mo/L (SCC) 0.04 54.7 Log 1/ Time Temp Log [ ] 13,20] (time) / [I] 199 20.06 23.0 30.04 0.04 0.04 80.0 22.8 45 40.02 0.04 79.0 21.6 50.08 0.03 51.0 22.4 60-080-02 95.0 23.4 7 0.08 0-01 1970 23.4 8 0.08 0.04 16.1 22.6arrow_forward(15 pts) Consider the molecule B2H6. Generate a molecular orbital diagram but this time using a different approach that draws on your knowledge and ability to put concepts together. First use VSEPR or some other method to make sure you know the ground state structure of the molecule. Next, generate an MO diagram for BH2. Sketch the highest occupied and lowest unoccupied MOs of the BH2 fragment. These are called frontier orbitals. Now use these frontier orbitals as your basis set for producing LGO's for B2H6. Since the BH2 frontier orbitals become the LGOS, you will have to think about what is in the middle of the molecule and treat its basis as well. Do you arrive at the same qualitative MO diagram as is discussed in the book? Sketch the new highest occupied and lowest unoccupied MOs for the molecule (B2H6).arrow_forwardQ8: Propose an efficient synthesis of cyclopentene from cyclopentane.arrow_forward
- Q7: Use compound A-D, design two different ways to synthesize E. Which way is preferred? Please explain. CH3I ONa NaOCH 3 A B C D E OCH3arrow_forwardPredict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forward(10 pts) The density of metallic copper is 8.92 g cm³. The structure of this metal is cubic close-packed. What is the atomic radius of copper in copper metal?arrow_forward
- Predict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forwardPredict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forwardQ3: Rank the following compounds in increasing reactivity of E1 and E2 eliminations, respectively. Br ca. go do A CI CI B C CI Darrow_forward
- Q5: Predict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2). H₂O דיי "Br KN3 CH3CH2OH NaNH2 NH3 Page 3 of 6 Chem 0310 Organic Chemistry 1 HW Problem Sets CI Br excess NaOCH 3 CH3OH Br KOC(CH3)3 DuckDuckGarrow_forwardQ4: Circle the substrate that gives a single alkene product in a E2 elimination. CI CI Br Brarrow_forwardPlease calculate the chemical shift of each protonsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning
Step by Step Stoichiometry Practice Problems | How to Pass ChemistryMole Conversions Made Easy: How to Convert Between Grams and Moles; Author: Ketzbook;https://www.youtube.com/watch?v=b2raanVWU6c;License: Standard YouTube License, CC-BY