
ORG CHEM CONNECT CARD
6th Edition
ISBN: 9781264860746
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 70P
Draw the products of each Lewis acid-base reaction. Label the electrophile and
nucleophile.
a.  c.
c. 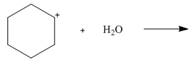
b. 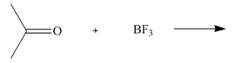
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
#1. Retro-Electrochemical Reaction: A ring has been made, but the light is causing the molecule to un-
cyclize. Undo the ring into all possible molecules. (2pts, no partial credit)
hv
Don't used Ai solution
I have a question about this problem involving mechanisms and drawing curved arrows for acids and bases. I know we need to identify the nucleophile and electrophile, but are there different types of reactions? For instance, what about Grignard reagents and other types that I might not be familiar with? Can you help me with this? I want to identify the names of the mechanisms for problems 1-14, such as Gilman reagents and others. Are they all the same? Also, could you rewrite it so I can better understand? The handwriting is pretty cluttered. Additionally, I need to label the nucleophile and electrophile, but my main concern is whether those reactions differ, like the "Brønsted-Lowry acid-base mechanism, Lewis acid-base mechanism, acid-catalyzed mechanisms, acid-catalyzed reactions, base-catalyzed reactions, nucleophilic substitution mechanisms (SN1 and SN2), elimination reactions (E1 and E2), organometallic mechanisms, and so forth."
Chapter 2 Solutions
ORG CHEM CONNECT CARD
Ch. 2.1 - a. Which compounds are Bronsted-Lowry acids:...Ch. 2.2 - a. Draw the conjugate acid of each base:...Ch. 2.2 - Label each statement as True or False.
a. is the...Ch. 2.2 - Decide which compound is the acid and which is the...Ch. 2.2 - Draw the products formed from the acid-base...Ch. 2.3 - Which compound in each pair is the stronger acid?...Ch. 2.3 - Use a calculator when necessary to answer the...Ch. 2.3 - Rank the conjugate bases of each of group of acids...Ch. 2.3 - Problem-2.10 Considers two acids: (formic acid,)...Ch. 2.3 - Prob. 11P
Ch. 2.4 - Draw the products of each reaction and determine...Ch. 2.4 - Prob. 13PCh. 2.5 - Without reference to a pKa table, decide which...Ch. 2.5 - Rank the labeled H atoms in the following compound...Ch. 2.5 - Which hydrogen in pseudoephedrine, the nasal...Ch. 2.5 - Which compound in each pair is the stronger acid?...Ch. 2.5 - Glycolic acid, HOCH2CO2H, is the simplest member...Ch. 2.5 - Explain the apparent paradox. HBr is a stronger...Ch. 2.5 - The CH bond in acetone, (CH3)2C=O, has a pKa of...Ch. 2.5 - Prob. 23PCh. 2.5 - For each pair of compounds: [1] Which indicated H...Ch. 2.5 - Rank the compounds in each group in order of...Ch. 2.5 - Prob. 26PCh. 2.5 - Prob. 27PCh. 2.6 - Prob. 28PCh. 2.7 - Problem 2.29
Compounds like amphetamine that...Ch. 2.8 - Problem 2.30 Which species are Lewis bases?
a. b....Ch. 2.8 - Which species are Lewis acids?
a. b. c. d.
Ch. 2.8 - For each reaction, label the Lewis acid and base....Ch. 2.8 - Prob. 33PCh. 2.8 - Prob. 34PCh. 2.8 - Label the Lewis acid and base. Use curved arrow...Ch. 2 - 2.36 Propranolol is an antihypertensive agent—that...Ch. 2 - 2.37 Amphetamine is a powerful stimulant of the...Ch. 2 - 2.38 What is the conjugate acid of each base?
a....Ch. 2 - 2.39 What is the conjugate base of each acid?
a....Ch. 2 - Draw the products of each proton transfer...Ch. 2 - Prob. 43PCh. 2 - What is Ka for each compound? Use a calculator...Ch. 2 - What is the pKa for each compound? a. b. c.Ch. 2 - Which of the following bases are strong enough to...Ch. 2 - Draw the products of each reaction. Use the pKa...Ch. 2 - a. What is the conjugate acid of A? b. What is the...Ch. 2 - Dimethyl ether (CH3OCH3) and ethanol (CH3CH2OH)...Ch. 2 - 2.59 Atenolol is a (beta) blocker, a drug used to...Ch. 2 - 2.60 Use the principles in Section 2.5 to label...Ch. 2 - 2.61 Label the three most acidic hydrogen atoms in...Ch. 2 - Prob. 66PCh. 2 - 2.63 Classify each compound as a Lewis base, a...Ch. 2 - 2.64 Classify each species as a Lewis acid, a...Ch. 2 - Label the Lewis acid and Lewis base in each...Ch. 2 - 2.66 Draw the products of each Lewis acid-base...Ch. 2 - Prob. 71PCh. 2 - 2.68 Answer the following questions about the four...Ch. 2 - Prob. 73PCh. 2 - 2.70 Hydroxide can react as a Brønsted-Lowry base...Ch. 2 - 2.71 Answer the following questions about esmolol,...Ch. 2 - Prob. 76PCh. 2 - Prob. 77PCh. 2 - Prob. 82P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. 200 LOD For an unknown compound with a molecular ion of 101 m/z: a. Use the molecular ion to propose at least two molecular formulas. (show your work) b. What is the DU for each of your possible formulas? (show your work) C. Solve the structure and assign each of the following spectra. 8 6 4 2 (ppm) 150 100 50 ō (ppm) 4000 3000 2000 1500 1000 500 HAVENUMBERI-11arrow_forwardComplete the spectroscopy with structurearrow_forwardComplete the spectroscopy with structurearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY