
Student Solutions Manual for Zumdahl/Zumdahl/DeCoste?s Chemistry, 10th Edition
10th Edition
ISBN: 9781305957510
Author: ZUMDAHL, Steven S.; Zumdahl, Susan A.; DeCoste, Donald J.
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 63E
Write the symbol of each atom using the
- a.
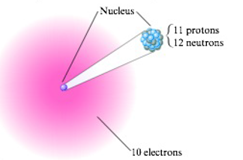
- b.
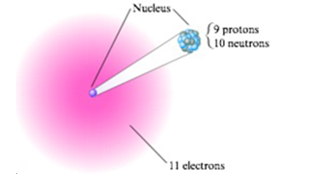
- c.
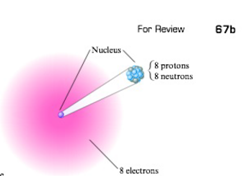
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Michael Reactions
19.52 Draw the products from the following Michael addition reactions.
1.
H&C CH
(a)
i
2. H₂O*
(b)
OEt
(c)
EtO
H₂NEt
(d)
ΕΙΟ
+
1. NaOEt
2. H₂O'
H
H
1. NaOEt
2. H₂O*
Rank the labeled protons (Ha-Hd) in order of increasing acidity, starting with the least acidic.
НОН НЬ
OHd
Онс
Can the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left?
?
starting
material
target
If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area.
Be sure you follow the standard ALEKS rules for submitting syntheses.
+ More...
Note for advanced students: you may assume that you are using a large excess of benzene as your starting material.
C
:0
T
Add/Remove step
G
Chapter 2 Solutions
Student Solutions Manual for Zumdahl/Zumdahl/DeCoste?s Chemistry, 10th Edition
Ch. 2 - Use Daltons atomic theory to account for each of...Ch. 2 - What evidence led to the conclusion that cathode...Ch. 2 - What discoveries were made by J. J. Thomson, Henri...Ch. 2 - Consider Ernest Rutherfords -particle bombardment...Ch. 2 - Do the proton and the neutron have exactly the...Ch. 2 - What is the distinction between atomic number and...Ch. 2 - Distinguish between the terms family and period in...Ch. 2 - The compounds AlCl3, CrCl3, and ICl3 have similar...Ch. 2 - Prob. 9RQCh. 2 - How would you name HBrO4, KIO3, NaBrO2, and HIO?...
Ch. 2 - Which of the following is true about an individual...Ch. 2 - How would you go about finding the number of chalk...Ch. 2 - These questions concern the work of J. J. Thomson....Ch. 2 - Prob. 4ALQCh. 2 - You have a chemical in a sealed glass container...Ch. 2 - The formula of water is If-O. Which of the...Ch. 2 - You may have noticed that when water boils, you...Ch. 2 - One of the best indications of a useful theory is...Ch. 2 - Prob. 9ALQCh. 2 - Label each of the following as an atomic element,...Ch. 2 - Why is the term sodium chloride molecule incorrect...Ch. 2 - Prob. 12ALQCh. 2 - Label each of the following as an atomic element,...Ch. 2 - Prob. 14ALQCh. 2 - Prob. 15ALQCh. 2 - Prob. 16ALQCh. 2 - Which of tire following explain how an ion is...Ch. 2 - What refinements had to be made in Daltons atomic...Ch. 2 - When hydrogen is burned in oxygen to form water,...Ch. 2 - The two most reactive families of elements are the...Ch. 2 - Explain the law of conservation of mass, the law...Ch. 2 - Section 2.3 describes the postulates of Daltons...Ch. 2 - The contributions of J. J. Thomson and Ernest...Ch. 2 - Prob. 24QCh. 2 - The number of protons in an atom determines the...Ch. 2 - If the volume of a proton were similar to the...Ch. 2 - Prob. 27QCh. 2 - List some characteristic properties that...Ch. 2 - Consider the elements of Group 4A (the carbon...Ch. 2 - Chlorine has two natural isotopes: 1737Cl and...Ch. 2 - Before an electrocardiogram (ECG) is recorded for...Ch. 2 - Distinguish between the following terms. a....Ch. 2 - Label the type of bonding for each of the...Ch. 2 - The vitamin niacin (nicotinic acid. C6H5NO2) can...Ch. 2 - Prob. 35QCh. 2 - Prob. 36QCh. 2 - When mixtures of gaseous H2 and gaseous Cl2 react,...Ch. 2 - Observations of the reaction between nitrogen gas...Ch. 2 - A sample of chloroform is found to contain 12.0 g...Ch. 2 - A sample of H2SO4 contains 2.02 g of hydrogen,...Ch. 2 - Consider 80.0-g samples of two different compounds...Ch. 2 - Several compounds containing sulfur and fluorine...Ch. 2 - The three most stable oxides of carbon ire carbon...Ch. 2 - Two elements. R and Q, combine to form two binary...Ch. 2 - In Section 1.1 of the text, the concept of a...Ch. 2 - In a combustion reaction, 46.0 g of ethanol reacts...Ch. 2 - Early tables of atomic weights (masses) were...Ch. 2 - Indium oxide contains 4.784 g of indium for every...Ch. 2 - Prob. 49ECh. 2 - If you wanted to make an accurate scale model of...Ch. 2 - In an experiment it was found that the total...Ch. 2 - A chemist in a galaxy tar, far away performed the...Ch. 2 - What are the symbols of the following nonmetals:...Ch. 2 - Prob. 55ECh. 2 - In the periodic table, how many elements are found...Ch. 2 - a. Classify the following elements as metals or...Ch. 2 - a. List the noble gas elements. Which of the noble...Ch. 2 - For each of the following sets of elements, label...Ch. 2 - Prob. 60ECh. 2 - Prob. 61ECh. 2 - Write the atomic symbol (ZAX) for each of the...Ch. 2 - Write the symbol of each atom using the ZAX...Ch. 2 - For carbon-14 and carbon-12, how many protons and...Ch. 2 - How many protons and neutrons are in the nucleus...Ch. 2 - Prob. 66ECh. 2 - For each of the following ions, indicate the...Ch. 2 - How many protons, neutrons, and electrons are in...Ch. 2 - Prob. 69ECh. 2 - What is the symbol of an ion with 16 protons, 18...Ch. 2 - Complete the following table: Symbol Number of...Ch. 2 - Complete the following table: Symbol Number of...Ch. 2 - Would you expect each of the following atoms to...Ch. 2 - Prob. 74ECh. 2 - Name the compounds in parts ad and write the...Ch. 2 - Name the compounds in parts a-d and write the...Ch. 2 - Prob. 77ECh. 2 - Prob. 78ECh. 2 - Prob. 79ECh. 2 - Prob. 80ECh. 2 - Prob. 81ECh. 2 - Prob. 82ECh. 2 - Prob. 83ECh. 2 - Prob. 84ECh. 2 - Prob. 85ECh. 2 - Prob. 86ECh. 2 - Prob. 87ECh. 2 - Write the formula for each of the following...Ch. 2 - Prob. 89ECh. 2 - Write the formula for each of the following...Ch. 2 - Prob. 91ECh. 2 - Each of the following compounds is incorrectly...Ch. 2 - Insulin is a complex protein molecule produced by...Ch. 2 - Carbohydrates, a class of compounds containing the...Ch. 2 - Prob. 95AECh. 2 - What are the symbols for the following nonmetal...Ch. 2 - Four Fe2+ ions are key components of hemoglobin,...Ch. 2 - Which of the following statements is(are) true?...Ch. 2 - The isotope of an unknown element, X, has a mass...Ch. 2 - Prob. 100AECh. 2 - Prob. 101AECh. 2 - Identify each of the following elements: a. a...Ch. 2 - Prob. 103AECh. 2 - Prob. 104AECh. 2 - Prob. 105AECh. 2 - Prob. 106AECh. 2 - Prob. 107AECh. 2 - Prob. 108AECh. 2 - Consider 100.0-g samples of two different...Ch. 2 - Give the systematic name for the following...Ch. 2 - Prob. 111CWPCh. 2 - Prob. 112CWPCh. 2 - Complete the following table. Atom/Ion Protons...Ch. 2 - Which of the following is{are) correct? a. 40Ca2...Ch. 2 - Prob. 115CWPCh. 2 - Prob. 116CWPCh. 2 - Prob. 117CWPCh. 2 - Prob. 118CWPCh. 2 - Reaction of 2.0 L of hydrogen gas with 1.0 L of...Ch. 2 - A combustion reaction involves the reaction of a...Ch. 2 - A chemistry instructor makes the following claim:...Ch. 2 - The early alchemists used to do an experiment in...Ch. 2 - Consider the chemical reaction as depicted below....Ch. 2 - Each of the following statements is true, but...Ch. 2 - You have two distinct gaseous compounds made from...Ch. 2 - A single molecule has a mass of 7.31 1023 g....Ch. 2 - You take three compounds, each consisting of two...Ch. 2 - Prob. 129IPCh. 2 - Prob. 130IPCh. 2 - Using the information in Table 2.1, answer the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The following equations represent the formation of compound MX. What is the AH for the electron affinity of X (g)? X₂ (g) → 2X (g) M (s) → M (g) M (g) M (g) + e- AH = 60 kJ/mol AH = 22 kJ/mol X (g) + e-X (g) M* (g) +X (g) → MX (s) AH = 118 kJ/mol AH = ? AH = -190 kJ/mol AH = -100 kJ/mol a) -80 kJ b) -30 kJ c) -20 kJ d) 20 kJ e) 156 kJarrow_forwardA covalent bond is the result of the a) b) c) d) e) overlap of two half-filled s orbitals overlap of a half-filled s orbital and a half-filled p orbital overlap of two half-filled p orbitals along their axes parallel overlap of two half-filled parallel p orbitals all of the abovearrow_forwardCan the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C T Add/Remove step X ноarrow_forward
- Which one of the following atoms should have the largest electron affinity? a) b) c) d) 으으 e) 1s² 2s² 2p6 3s¹ 1s² 2s² 2p5 1s² 2s² 2p 3s² 3p² 1s² 2s 2p 3s² 3p6 4s2 3ds 1s² 2s² 2p6arrow_forwardAll of the following are allowed energy levels except _. a) 3f b) 1s c) 3d d) 5p e) 6sarrow_forwardA student wants to make the following product in good yield from a single transformation step, starting from benzene. Add any organic reagents the student is missing on the left-hand side of the arrow, and any addition reagents that are necessary above or below the arrow. If this product can't be made in good yield with a single transformation step, check the box below the drawing area. Note for advanced students: you may assume that an excess of benzene is used as part of the reaction conditions. : ☐ + I X This product can't be made in a single transformation step.arrow_forward
- Ppplllleeeaaasssseeee helllppp wiithhh thisss Organic chemistryyyyyy I talked like this because AI is very annoyingarrow_forwardName the family to which each organic compound belongs. The first answer has been filled in for you. compound CH₂ || CH3-C-NH2 0 ။ CH3-C-CH₂ CH=O–CH=CH, CH₂ HO CH2-CH2-CH-CH3 family amine Darrow_forward1b. Br LOHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHERChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHERChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning



Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Atomic Number, Atomic Mass, and the Atomic Structure | How to Pass ChemistryThe Nucleus: Crash Course Chemistry #1; Author: Crash Course;https://www.youtube.com/watch?v=FSyAehMdpyI;License: Standard YouTube License, CC-BY