
Loose Leaf for General, Organic and Biological Chemistry with Connect 2 Year Access Card
4th Edition
ISBN: 9781260269284
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 50P
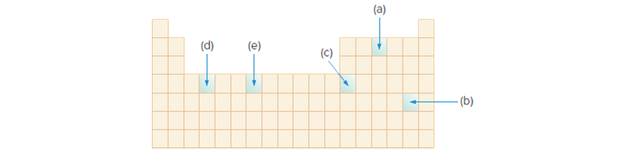
Use an orbital diagram to write the electronic configuration of each element whose location is shown in the periodic table.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the complete mechanism for the reaction below. Please include appropriate arrows, intermediates, and formal charges.
(c) The following data have been obtained for the hydrolysis of sucrose, C12H22O11, to
glucose, C6H12O6, and fructose C6H12O6, in acidic solution:
C12H22O11 + H2O → C6H12O6 + C6H12O6
[sucrose]/mol dm³
t/min
0
0.316
14
0.300
39
0.274
60
0.256
80
0.238
110
0.211
(i) Graphically prove the order of the reaction and determine the rate constant of the
reaction.
(ii) Determine the half-life, t½ for the hydrolysis of sucrose.
(III) adsorbent
(b) Adsorption of the hexacyanoferrate (III) ion, [Fe(CN)6] ³, on y-Al2O3 from aqueous
solution was examined. The adsorption was modelled using a modified Langmuir
isotherm, yielding the following values of Kat pH = 6.5:
(ii)
T/K
10-10 K
280
2.505
295
1.819
310
1.364
325
1.050
Determine the enthalpy of adsorption, AadsHⓇ.
If the reported value of entropy of adsorption, Aads Se = 146 J K-1 mol-1 under the above
conditions, determine Aads Gº.
Chapter 2 Solutions
Loose Leaf for General, Organic and Biological Chemistry with Connect 2 Year Access Card
Ch. 2.1 - Give the symbol for each element. a. calcium, a...Ch. 2.1 - Give the name corresponding to each element...Ch. 2.1 - Locate each element in the periodic table and...Ch. 2.1 - Prob. 2.4PCh. 2.1 - Identify the elements used ineach example of...Ch. 2.1 - Identify the elements in each chemical formula,...Ch. 2.1 - Prob. 2.6PCh. 2.1 - Prob. 2.2PPCh. 2.2 - Prob. 2.3PPCh. 2.2 - Prob. 2.4PP
Ch. 2.2 - For the given atom: (a) determine the number of...Ch. 2.2 - How many protons, neutrons, and electrons are...Ch. 2.2 - What is the mass number of an atom that contains...Ch. 2.3 - For each atom give the following information: [1]...Ch. 2.3 - Write an isotope symbol for the isotope of...Ch. 2.3 - Magnesium has three isotopes that contain 12, 13,...Ch. 2.3 - Prob. 2.9PPCh. 2.3 - Calculate the atomic weight of each element given...Ch. 2.4 - Prob. 2.9PCh. 2.4 - Label each macronutrient in Figure 2.2 in the...Ch. 2.4 - Identify the element fitting each description. an...Ch. 2.4 - Identify each highlighted element in the periodic...Ch. 2.5 - How many electrons are present in each shell,...Ch. 2.6 - What element has each electronic configuration? a....Ch. 2.6 - What element(s) in the first and second period fit...Ch. 2.6 - Draw an orbital diagram for each element; (a)...Ch. 2.6 - Give the electronic configuration for each element...Ch. 2.7 - Prob. 2.13PPCh. 2.7 - Determine the number of valence electrons and give...Ch. 2.7 - Give the electron-dot symbol for each element: (a)...Ch. 2.8 - Which element in each pair has the larger atomic...Ch. 2.8 - Prob. 2.16PCh. 2.8 - Prob. 2.17PPCh. 2.8 - (a) Which of the indicated atoms has the smaller...Ch. 2 - Identify the elements used in each example of...Ch. 2 - Write a chemical formula for each example of...Ch. 2 - Give the name of the elements in each group of...Ch. 2 - What element(s) are designated by each symbol or...Ch. 2 - Does each chemical formula represent an element or...Ch. 2 - Identify the elements in each chemical formula and...Ch. 2 - Prob. 23PCh. 2 - Prob. 24PCh. 2 - Give all of the terms that apply to each...Ch. 2 - Give all of the terms that apply to each...Ch. 2 - Give the following information about the atom...Ch. 2 - Give the following information about the atom...Ch. 2 - Prob. 29PCh. 2 - Prob. 30PCh. 2 - Prob. 31PCh. 2 - Consider the four atoms-L, M, N, and X- with the...Ch. 2 - Label each region on the periodic table. Noble...Ch. 2 - Identify each highlighted element in the periodic...Ch. 2 - Prob. 35PCh. 2 - Prob. 36PCh. 2 - Prob. 37PCh. 2 - Prob. 38PCh. 2 - Prob. 39PCh. 2 - Complete the followin table for the two most...Ch. 2 - How many protons, neutrons, and electrons are...Ch. 2 - Give the number of protons, neutrons, and...Ch. 2 - Write the element symbol that fits each...Ch. 2 - Write the element symbol that fits each...Ch. 2 - Calculate the atomic weight of silver, which has...Ch. 2 - Calculate the atomic weight of antimony, which has...Ch. 2 - Prob. 47PCh. 2 - What is the maximum number of electrons that can...Ch. 2 - Prob. 49PCh. 2 - Use an orbital diagram to write the electronic...Ch. 2 - Prob. 51PCh. 2 - For each element in Problem 2.50: (a) Write out...Ch. 2 - Prob. 53PCh. 2 - Prob. 54PCh. 2 - Give the total number of electrons, the number of...Ch. 2 - Give the total number of electrons, the number of...Ch. 2 - Prob. 57PCh. 2 - Prob. 58PCh. 2 - Prob. 59PCh. 2 - Prob. 60PCh. 2 - Prob. 61PCh. 2 - Which of the following orbital diagrams are...Ch. 2 - Prob. 63PCh. 2 - Prob. 64PCh. 2 - Prob. 65PCh. 2 - Write an electron-dot symbol for each element: (a)...Ch. 2 - Prob. 67PCh. 2 - Prob. 68PCh. 2 - Prob. 69PCh. 2 - Prob. 70PCh. 2 - Prob. 71PCh. 2 - For each pair of elements in Problem 2.70, label...Ch. 2 - Rank the atoms in each group in order of...Ch. 2 - Prob. 74PCh. 2 - Prob. 75PCh. 2 - Prob. 76PCh. 2 - Prob. 77PCh. 2 - Prob. 78PCh. 2 - Prob. 79PCh. 2 - (a) What is the chemical formula for...Ch. 2 - Answer the following questions about the...Ch. 2 - Platinum is a precious metal used in a wide...Ch. 2 - Prob. 83PCh. 2 - Answer the following questions about the...Ch. 2 - Prob. 85CPCh. 2 - Prob. 86CP
Additional Science Textbook Solutions
Find more solutions based on key concepts
An obese 55-year-old woman consults her physician about minor chest pains during exercise. Explain the physicia...
Biology: Life on Earth with Physiology (11th Edition)
Give the IUPAC name for each compound.
Organic Chemistry
What are the cervical and lumbar enlargements?
Principles of Anatomy and Physiology
Sea turtles have disappeared from many regions, and one way of trying to save them is to reintroduce them to ar...
MARINE BIOLOGY
Gregor Mendel never saw a gene, yet he concluded that some inherited factors were responsible for the patterns ...
Campbell Essential Biology (7th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. Propose an efficient synthesis for each of the following transformations. Pay careful attention to both the regio and stereochemical outcomes. ¡ H H racemicarrow_forwardZeroth Order Reaction In a certain experiment the decomposition of hydrogen iodide on finely divided gold is zeroth order with respect to HI. 2HI(g) Au H2(g) + 12(9) Rate = -d[HI]/dt k = 2.00x104 mol L-1 s-1 If the experiment has an initial HI concentration of 0.460 mol/L, what is the concentration of HI after 28.0 minutes? 1 pts Submit Answer Tries 0/5 How long will it take for all of the HI to decompose? 1 pts Submit Answer Tries 0/5 What is the rate of formation of H2 16.0 minutes after the reaction is initiated? 1 pts Submit Answer Tries 0/5arrow_forwardangelarodriguezmunoz149@gmail.com Hi i need help with this question i am not sure what the right answers are.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

The Bohr Model of the atom and Atomic Emission Spectra: Atomic Structure tutorial | Crash Chemistry; Author: Crash Chemistry Academy;https://www.youtube.com/watch?v=apuWi_Fbtys;License: Standard YouTube License, CC-BY