
Biology 2e
2nd Edition
ISBN: 9781947172517
Author: Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 3VCQ
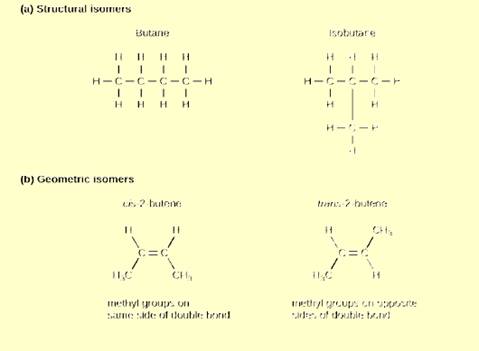
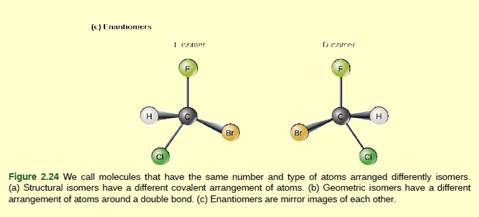
Figure 2.24 Which of the following statements is
false?
- Molecules with the formulas CH3CH2COOH and C3H6O2 could be structural isomers.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
I have the first half finished... just need the bottom half.
13. Practice Calculations: 3 colonies were suspended in the following dilution series and then a
viable plate count and microscope count was performed. Calculate IDF's, TDF's and then
calculate the CFU/mL in each tube by both methods. Finally calculate the cells in 1 colony by
both methods. Show all of your calculations in the space provided on the following pages.
3 colonies
56
cells
10 μL
10 μL
100 μL
500 με
m
OS
A
B
D
5.0 mL
990 με
990 με
900 με
500 μL
EN
2
100 με
100 μL
118
colonies
12
colonies
Describe and give a specific example of how successionary stage is related to species diversity?
Chapter 2 Solutions
Biology 2e
Ch. 2 - Figure 2.3 How many neutrons do carbon-12 and...Ch. 2 - Figure 2.7 An atom may give, take, or share...Ch. 2 - Figure 2.24 Which of the following statements is...Ch. 2 - If xenon has an atomic number of 54 and a mass...Ch. 2 - Atoms that vary in the number of neutrons found in...Ch. 2 - Potassium has an atomic number of 19. What is its...Ch. 2 - Which type of bond represents a weak chemical...Ch. 2 - Which of the following statements is not true?...Ch. 2 - When acids are added to a solution, the pH should...Ch. 2 - We call a molecule that binds up excess hydrogen...
Ch. 2 - Which of the following statements is true? Acids...Ch. 2 - Each carbon molecule can bond with as many as...Ch. 2 - Which of the following is not a functional group...Ch. 2 - What makes ionic bonds different from covalent...Ch. 2 - Why are hydrogen bonds and van der Waals...Ch. 2 - Discuss how buffers help prevent drastic swings in...Ch. 2 - Why can some insects walk on water?Ch. 2 - What property of carbon makes it essential for...Ch. 2 - Compare and contrast saturated and unsaturated...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Use a globe or map to determine, as accurately as possible, the latitude and longitude of Athens, Greece.
Applications and Investigations in Earth Science (9th Edition)
Which type of cartilage is most plentiful in the adult body?
Anatomy & Physiology (6th Edition)
What are the two types of bone marrow, and what are their functions?
Human Anatomy & Physiology (2nd Edition)
16. Explain some of the reasons why the human species has been able to expand in number and distribution to a g...
Campbell Biology: Concepts & Connections (9th Edition)
30. Drosophila has a diploid chromosome number of 2n = 8, which includes one pair of sex chromosomes (XX in fem...
Genetic Analysis: An Integrated Approach (3rd Edition)
Police Captain Jeffers has suffered a myocardial infarction. a. Explain to his (nonmedically oriented) family w...
Human Physiology: An Integrated Approach (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- Explain down bellow what happens to the cell in pictures not in words: Decreased pH in mitochondria Increased ATP Decreased pH in cytosol Increased hydrolysis Decreasing glycogen and triglycerides Increased MAP kinase activity Poor ion transport → For each one:→ What normally happens?→ What is wrong now?→ How does it mess up the cell?arrow_forward1.) Community Diversity: The brown and orange line represent two different plant communities. a. Which color represents the community with a higher species richness? b. Which color represents the community with a higher species evenness? Relative abundance 0.1 0.04 0.001 2 4 6 8 10 12 14 16 18 20 22 24 Rank abundance c. What is the maximum value of the Simpson's diversity index (remember, Simpson's index is D = p², Simpson's diversity index is 1-D)? d. If the Simpson's diversity index equals 1, what does that mean about the number of species and their relative abundance within community being assessed?arrow_forward1.) Community Diversity: The brown and orange line represent two different plant communities. a. Which color represents the community with a higher species richness? b. Which color represents the community with a higher species evenness? Relative abundance 0.1 0.04 0.001 2 4 6 8 10 12 14 16 18 20 22 24 Rank abundance c. What is the maximum value of the Simpson's diversity index (remember, Simpson's index is D = p², Simpson's diversity index is 1-D)? d. If the Simpson's diversity index equals 1, what does that mean about the number of species and their relative abundance within community being assessed?arrow_forward
- what measures can a mother to take to improve the produce of her to milk to her newborn baby ?arrow_forward1. Color the line that represents all ancestors of the Eastern white pine tree green (but only the ancestral line NOT shared with other organisms) 2. Oncle the last common ancestor of the Colorado blue spruce tree and Eastern white pine tree. 3. Put a box around the last common ancestor of the sugar maple tree and the dogwood tree. 4. Put a triangle around the last common ancestor of the red pine tree and the american holly bush. 5. Color the line that represents all ancestors of the Ponderosa pine tree red (including all shared ancestors). 6. Color the line that represents all ancestors of the American elm tree blue (including all shared ancestors). 7 Color the line that represents all ancestors of the Sabal palm tree purple (including all shared ancestors) 8. Using a yellow highlighter or colored pencil, circle the clade that includes all pine trees. 9. Using a orange highlighter or colored pencil, circle the clade that includes all gymnosperms 10. Can you tell…arrow_forwardYou have been hired as a public relations specialist to give invertebrates a good name. After all, they are much more than just creepy crawly bugs! Your first task though is to convince yourself that is true. The best way to do that is to start close to home. Find something in your house that is a product obtained directly from an invertebrate or only due to an invertebrate’s actions. Describe the product, its function and utility, as well as any human manufactured alternatives. Be sure to highlight the advantages of obtaining this directly from nature. Keep in mind, a product can be something you use, wear, eat, or enjoy for its visual appeal.arrow_forward
- Use the following tree diagram to answer Questions #8-10. 8) Which of the following two animals are the most closely related based on the tree to the left? a) Pig and camel b) Hippo and pig c) Deer and cow 9) CIRCLE on the tree diagram where the common ancestor between a hippo and a cow is. 10) Put a SQUARE on the tree diagram where the common ancestor between a pig and a peccary is.arrow_forwardExplain: Healthy Cell Function Overview→ Briefly describe how a healthy cell usually works: metabolism (ATP production), pH balance, glycogen storage, ion transport, enzymes, etc. Gene Mutation and Genetics Part→ Focus on the autosomal recessive mutation and explain: How gene mutation affects the cell. How autosomal inheritance works. Compare the normal and mutated gene sequences simply. → Talk about possible consequences of a faulty hydrolytic enzyme.arrow_forwardCan you fill out those termsarrow_forward
- Explain down bellow what happens to the cell: Decreased pH in mitochondria Increased ATP Decreased pH in cytosol Increased hydrolysis Decreasing glycogen and triglycerides Increased MAP kinase activity Poor ion transport → For each one:→ What normally happens?→ What is wrong now?→ How does it mess up the cell?arrow_forwardAn 1100 pound equine patient was given 20 mg/kg sucralfate 3 times a day, 2.8 mg/kg famotidine twice a day, and 10mg/kg doxycycline twice a day. Sucralfate comes as a 1 gm tablet, famotidine as 20 mg tablets, and doxycycline as 100mg tablets. All are in bottles of 100 tablets.How many total mg are needed for the patient and how many tablets of each would be needed to provide each dose?How many bottles of each would be needed to have available if this patient were to be on this drug regimen for 5 days?arrow_forwardThe patient needs a solution of 2.5% dextrose in Lactated Ringer’s solution to run at 75 ml/hr for at least the next 12hours. LRS comes in fluid bags of 500 ml, 1 Liter, 3 Liters and 5 Liters. How can a 2.5% solution be made by adding50% dextrose to the LRS?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College

Human Biology (MindTap Course List)
Biology
ISBN:9781305112100
Author:Cecie Starr, Beverly McMillan
Publisher:Cengage Learning

Biology Today and Tomorrow without Physiology (Mi...
Biology
ISBN:9781305117396
Author:Cecie Starr, Christine Evers, Lisa Starr
Publisher:Cengage Learning


Biology (MindTap Course List)
Biology
ISBN:9781337392938
Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:Cengage Learning

Anatomy & Physiology
Biology
ISBN:9781938168130
Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:OpenStax College

Macromolecules | Classes and Functions; Author: 2 Minute Classroom;https://www.youtube.com/watch?v=V5hhrDFo8Vk;License: Standard youtube license