
Concept explainers
Write a balanced chemical equation for the combustion of each of the following compounds:
a) Decane
b) Cyclodecane
c) Methylcyclononane
d) Cyclopentylcyclopentane
Interpretation:
A balanced chemical equation for the combustion of each of the given compounds is to be written.
Concept introduction:
Alkanes are inert in acid-base reactions but undergo oxidation-reduction reactions.
During combustion, alkanes undergo oxidation.
This combustion reaction of alkanes is exothermic, and the products formed are carbon dioxide and water.
When balancing the reaction, the carbon and hydrogen is balanced first, leaving oxygen for the last to balance.
Answer to Problem 39P
Solution:
a)
b)
c)
d)
Explanation of Solution
a) Decane
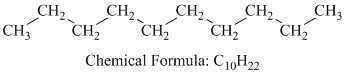
Decane is a straight chain alkane having the molecular formula
The combustion reaction of decane is written as follows:
To balance this reaction, first, the C and H are balanced.
Oxygen is balanced as follows:
The coefficients are converted to whole numbers as follows:
This is the complete balanced reaction of decane.
b) Cyclodecane
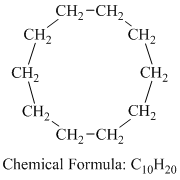
Cyclodecane is a cyclic alkane having the molecular formula
The combustion reaction of cyclodecane is written as follows:
To balance this reaction, first, the C and H are balanced.
Oxygen is balanced as follows:
This is the complete balanced reaction of cyclodecane.
c) Methylcyclononane
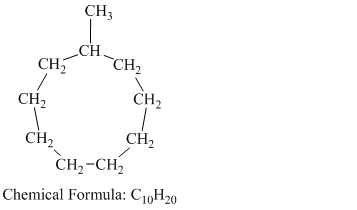
Methylcyclononane is a branched chain alkane having the molecular formula
The combustion reaction of methylcyclononane is written as follows:
To balance this reaction, first, the C and H are balanced.
Oxygen is balanced as follows:
This is the complete balanced reaction of methylcyclononane.
d) Cyclopentylcyclopentane
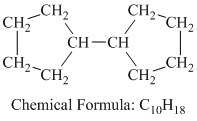
Cyclopentylcyclopentane is a branched chain alkane having the molecular formula
The combustion reaction of Cyclopentylcyclopentane is written as follows:
To balance this reaction, first, the C and H balanced.
Oxygen is balanced as follows:
The coefficients are converted to whole numbers as follows:
This is the complete balanced reaction of cyclopentylcyclopentane.
Want to see more full solutions like this?
Chapter 2 Solutions
ORGANIC CHEMISTRY (LOOSELEAF)-PACKAGE
- Rel. Intensity Q 1. Which one of the following is true of the compound whose mass spectrum is shown here? Explain how you decided. 100 a) It contains chlorine. b) It contains bromine. c) It contains neither chlorine nor bromine. 80- 60- 40- 20- 0.0 0.0 TT 40 80 120 160 m/z 2. Using the Table of IR Absorptions how could you distinguish between these two compounds in the IR? What absorbance would one compound have that the other compound does not? HO CIarrow_forwardIllustrate reaction mechanisms of alkenes with water in the presence of H2SO4, detailing each step of the process. Please show steps of processing. Please do both, I will thumb up for sure #1 #3arrow_forwardDraw the following molecule: (Z)-1-chloro-1-butenearrow_forward
- Identify the molecule as having a(n) E, Z, cis, or trans configuration. CH3 H₁₂C ○ E ○ z ○ cis transarrow_forwardIdentify the molecule as having a(n) E, Z, cis, or trans configuration. H₂C- CH3 О Е ○ cis ○ transarrow_forwardThe decomposition of dinitrogen pentoxide according to the equation: 50°C 2 N2O5(g) 4 NO2(g) + O2(g) follows first-order kinetics with a rate constant of 0.0065 s-1. If the initial concentration of N2O5 is 0.275 M, determine: the final concentration of N2O5 after 180 seconds. ...arrow_forward
- Don't used hand raitingarrow_forwardCS2(g) →CS(g) + S(g) The rate law is Rate = k[CS2] where k = 1.6 × 10−6 s−¹. S What is the concentration of CS2 after 5 hours if the initial concentration is 0.25 M?arrow_forwardCS2(g) → CS(g) + S(g) The rate law is Rate = k [CS2] where k = 1.6 × 10-6 s−1. S Calculate the half-life.arrow_forward
- The following is a first order reaction where the rate constant, k, is 6.29 x 10-3 min-*** What is the half-life? C2H4 C2H2 + H2arrow_forwardControl Chart Drawing Assignment The table below provides the number of alignment errors observed during the final inspection of a certain model of airplane. Calculate the central, upper, and lower control limits for the c-chart and draw the chart precisely on the graph sheet provided (based on 3-sigma limits). Your chart should include a line for each of the control limits (UCL, CL, and LCL) and the points for each observation. Number the x-axis 1 through 25 and evenly space the numbering for the y-axis. Connect the points by drawing a line as well. Label each line drawn. Airplane Number Number of alignment errors 201 7 202 6 203 6 204 7 205 4 206 7 207 8 208 12 209 9 210 9 211 8 212 5 213 5 214 9 215 8 216 15 217 6 218 4 219 13 220 7 221 8 222 15 223 6 224 6 225 10arrow_forwardCollagen is used to date artifacts. It has a rate constant = 1.20 x 10-4 /years. What is the half life of collagen?arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





