
Organic Chemistry - Standalone book
10th Edition
ISBN: 9780073511214
Author: Francis A Carey Dr., Robert M. Giuliano
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 30P
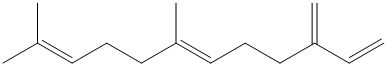
Aphids secrete an alarm pheromone having the structure shown. What is its molecular formula? Classify each carbon according to its hybridization state.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The structure of compound 1,1,2-trichloropropane is given below.
Cl
Cl
Cl
1
How many signals would you expect to find in the 'H NMR spectrum of 1,1,2-trichloropropane?
×
1,
How many signals do you expect in the H NMR spectrum for this molecule?
Write the answer below.
Also, in each of the drawing areas below is a copy of the molecule, with Hs shown. In each copy, one of the H atoms is colored red. Highlight in red all other H
atoms that would contribute to the same signal as the H already highlighted red.
Note for advanced students: In this question, any multiplet is counted as one signal.
Number of signals in the 'H NMR spectrum.
For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to
the same signal as the H atom already highlighted red.
If no other H atoms will contribute, check the box at right.
No additional Hs to color in top
molecule
For the molecule in the bottom drawing area, highlight in red any other H atoms that will contribute.
to the same signal as the H atom already highlighted red.
If no other H atoms will contribute, check the box at right.
No additional Hs to color in bottom
molecule
Check…
Incorrect
Row 2: Your answer is incorrect.
Consider this molecule:
How many H atoms are in this molecule?
22
How many different signals could be found in its 'H NMR spectrum?
12
Note: A multiplet is considered one signal.
Chapter 2 Solutions
Organic Chemistry - Standalone book
Ch. 2.4 - Prob. 1PCh. 2.7 - Prob. 2PCh. 2.8 - Identify the orbital overlaps of all of the bonds...Ch. 2.9 - The hydrocarbon shown, called vinylacetylene, is...Ch. 2.12 - Prob. 5PCh. 2.12 - Prob. 6PCh. 2.13 - Prob. 7PCh. 2.14 - Refer to Table 2.2 as needed to answer the...Ch. 2.15 - Prob. 9PCh. 2.15 - Prob. 10P
Ch. 2.16 - Prob. 11PCh. 2.17 - Prob. 12PCh. 2.18 - Prob. 13PCh. 2.20 - Prob. 14PCh. 2.21 - Match the boiling points with the appropriate...Ch. 2.22 - Write a balanced chemical equation for the...Ch. 2.22 - Using the data in Table 2.3, estimate the heat of...Ch. 2.22 - Prob. 18PCh. 2.22 - Prob. 19PCh. 2.23 - Prob. 20PCh. 2.23 - Which of the following reactions requires an...Ch. 2 - The general molecular formula for alkanes is...Ch. 2 - Prob. 23PCh. 2 - Prob. 24PCh. 2 - Prob. 25PCh. 2 - What is the hybridization of each carbon in...Ch. 2 - Prob. 27PCh. 2 - Does the overlap of two p orbitals in the fashion...Ch. 2 - Prob. 29PCh. 2 - Aphids secrete an alarm pheromone having the...Ch. 2 - All the parts of this problem refer to the alkane...Ch. 2 - Prob. 32PCh. 2 - Prob. 33PCh. 2 - Prob. 34PCh. 2 - From among the 18 constitutional isomers of C8H18,...Ch. 2 - Give the IUPAC name for each of the following...Ch. 2 - Using the method outlined in Section 2.16, give an...Ch. 2 - Prob. 38PCh. 2 - Write a balanced chemical equation for the...Ch. 2 - The heats of combustion of methane and butane are...Ch. 2 - In each of the following groups of compounds,...Ch. 2 - Given H for the reaction H2(g)+12O2(g)H2O(l)...Ch. 2 - Prob. 43PCh. 2 - Prob. 44PCh. 2 - Prob. 45PCh. 2 - Prob. 46PCh. 2 - Prob. 47PCh. 2 - Compound A undergoes the following reactions:...Ch. 2 - Prob. 49PCh. 2 - Some Biochemical Reactions of Alkanes Alkanes...Ch. 2 - Prob. 51DSPCh. 2 - Some Biochemical Reactions of Alkanes Alkanes...Ch. 2 - Prob. 53DSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 13 How many signals would you expect to see in the Check O signal(s) X § 'C NMR spectrum for the following compound? © 2025 McGraw Hillarrow_forward13 Consider the "C NMR spectrum below. 140 120 100 80 60 40 20 20 PPM 0 The spectrum belongs to which one of the following constitutional isomers of the compound C,H12? Select the single best answer. Check ✓ G Save For Later 2025 McGraw Hill LLC. All Rights Reserved. Terms of Usearrow_forwardThe structure of compound 1,3,5-trimethylbenzene (mesitylene) is given below. How many signals would you expect to find in the 'H NMR spectrum of 1,3,5-trimethylbenzene (mesitylene)? Check ×arrow_forward
- 1 How many signals do you expect in the 'H NMR spectrum for this molecule? CI CI Cl Write the answer in the table below. Also, in each of the drawing areas below is a copy of the molecule, with H atoms shown. In each copy, one of the H atoms is highlighted red. Highlight in red all other H atoms that would contribute to the same signal as the H already highlighted red. Note for advanced students: Remember, a multiplet is considered one signal in the 'H NMR spectrum. 1 Number of signals in the 'H NMR spectrum. ☐ For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. No additional H atoms to highlight in top molecule For the molecule in the bottom drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at…arrow_forwardwrtie the balanced equation and find the E° when the following half- reactions are combined Zn2+(aq) + 2e---> Zn(s) E°= -0.763V Ag+(aq) + e---> Ag (s) E°=+0.799Varrow_forwardConsider this molecule: How many H atoms are in this molecule? How many different signals could be found in its 'H NMR spectrum? Note: A multiplet is considered one signal. ☐arrow_forward
- Study this 'H NMR spectrum, and then answer the questions about it in the table below. Check 1.0- 0.5- 0.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0 What unit symbol should be written on the horizontal axis? What is the chemical shift & of the doublet? If there is no doublet, just check the box instead. Give your answer to 2 significant digits. What is the chemical shift of the signal immediately upfield of the doublet? If there is no doublet, or no signal upfield of it, check the box instead. What is the chemical shift & of the least deshielded proton? If you can't tell without more information, check the box instead. 血 8 = ☐ There is no doublet. 8 = ☐ No such signal. 8 = 0 Need more information.arrow_forwardhow many moles of H2O2 are required to react with 11g of N2H4 according to the following reaction? (atomic weights: N=14.01, H=1.008, O= 16.00) 7H2O2 + N2H4 -> 2HNO3 + 8H20arrow_forwardcalculate the number of moles of H2 produced from 0.78 moles of Ga and 1.92 moles HCL? 2Ga+6HCL->2GaCl3+3H2arrow_forward
- an adult human breathes 0.50L of air at 1 atm with each breath. If a 50L air tank at 200 atm is available, how man y breaths will the tank providearrow_forwardWhat are the advantages and/or disadvantages of using the MOHR titration method & AOEC method?arrow_forwardAre there any alternative methods better than the MOHR titration to quantitatively determine salt in a sample?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning


General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Types of bonds; Author: Edspira;https://www.youtube.com/watch?v=Jj0V01Arebk;License: Standard YouTube License, CC-BY